INTRODUCTION
Tuberculosis (TB) is one of the most prevalent diseases worldwide. It has been estimated that up to 25% of the global population was infected by Mycobacterium tuberculosis by 2014 1. In 2019, 10 million people developed this disease, and approximately 1.2 million died from it. As a result, TB is ranked among the ten diseases with the highest mortality and is the primary cause of death due to a single infectious agent, surpassing HIV/AIDS 2. Immunosuppression, socioeconomic factors, nutritional status, and substance abuse are risk factors for disease development (3, 4). The latter is the most common behavioral risk factor in the United States 5.
The World Health Organization (WHO) has proposed the "End TB" strategy to eradicate tuberculosis by 2035 through prevention and comprehensive patient-centered care (6, 7). However, drug-resistant strains remain a significant challenge to achieving this goal. The primary cause of drug-resistant strains is loss to follow up during treatment, which has been associated with psychiatric disorders, specifically to substance use disorders 8.
There is a variable prevalence (10-59%) of latent TB among illicit drug users (5). In Brazil, 58 % of crack users have been found to have latent TB 9. Moreover, crack use appears to be an independent risk factor for the development of TB due to pulmonary damage caused by inhaled substances 10. Drug use (marijuana, cocaine, and terokal) has also been identified as a risk factor for relapse in patients who have initiated TB treatment 11. Cocaine and its derivatives are the principal stimulant drugs used in America and Western Europe, with 19 million users worldwide in 2018 12; a Brazilian study found a strong association between cocaine/crack use and loss to follow up during TB treatment 13.
Several studies have found a relationship between drug use of alcohol or illegal drugs and negative outcomes during TB treatment, but specific drug-related studies that synthesize the current evidence are still necessary (13, 14). This review aims to synthesize the available data on the association between cocaine-derived drug consumption and adverse outcomes of TB treatment (death, loss to follow-up, and treatment failure), answering the research question: Is the use of cocaine-derived drugs associated to adverse outcomes of TB treatment, specifically death, loss to follow-up, and treatment failure?
MATERIALS AND METHODS
Search strategy and selection criteria
We conducted a systematic review and meta-analysis following the PRISMA Statement guidelines (S1 checklist) to report our findings. The study protocol was registered in the PROSPERO database (CRD42021230010).
The PRISMA Statement is a widely used reporting guideline for systematic reviews and meta-analyses. It provides a checklist of items that should be included in the report to ensure transparency and reproducibility.
We searched for studies that evaluated, directly or indirectly, the association between illicit drug use of cocaine-derived substances and negative outcomes of TB treatment as defined by the WHO (death, loss to follow-up, and treatment failure) or as defined by the individual studies 15. Cross-sectional, case-control, cohort, and clinical trial studies were included in this review. We considered both latent and active tuberculosis (pulmonary or extrapulmonary) with any grade of drug-resistance diagnosed according to the criteria used by each study.
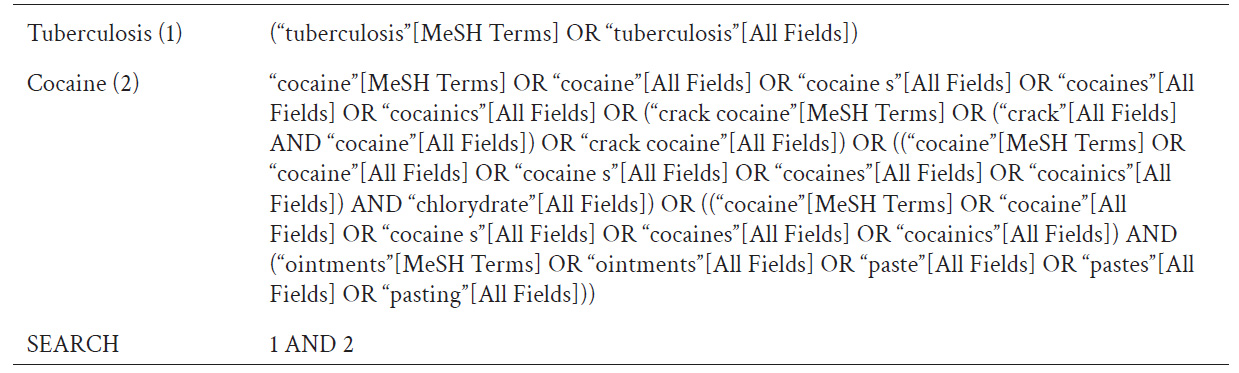
The electronic databases used were PubMed, Lilacs, EBSCOhost, Ovid, and Google Scholar for articles written in English and Spanish published up to December 31, 2020. Gray literature was not considered. The search strategy included the terms "Cocaine" and "Tuberculosis". Detailed search strategies for PubMed are described in Table 1.
Ethical approval was obtained from the Universidad Peruana Cayetano Heredia Integrated Unit of Investigation Management, Science, and Technology (SIDISI: 205425).
Data extraction and quality assessment
After eliminating duplicates, all identified articles were randomly assigned to two groups of two evaluators (FC & DT and CS & RL) who independently appraised each title and abstract using Rayyan's software blinding tool. Non eligible articles were eliminated. Using the same strategy, we proceeded to the full-text review to determine if they met the inclusion criteria. We registered each article included in a database through Google Forms, extracting relevant information: authors, year of publication, study type, patients' age, type of tuberculosis, illicit drugs use definition, and negative outcomes definition. No double entry by independent observers was used during this step. Disagreements in the decision to include an article were resolved by consensus or by a fifth reviewer (PR).
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of cohort and case-control studies. Clinical trials (16, 17) were evaluated with the NOS for cohorts because drug consumption was not an intervention of the investigators nor was it randomized among the population, but rather an exposure variable not included in the main research question, and thus the randomization.
Data analysis
A random effects model was used to estimate summary odds ratios (ORs) with 95% confidence intervals using the metaan command in STATA 16. A random effects model is appropriate when there is heterogeneity among the studies, which is often the case in meta-analyses.
The Cochrane Q test and the I2 statistic were used to evaluate the heterogeneity of the effects. The Cochrane Q test assesses whether there is significant heterogeneity among the studies, with a null hypothesis of no heterogeneity. The I2 statistic measures the percentage of variation in the effects that is due to heterogeneity rather than chance. Scores of 0.5 to 1 are indicative of high heterogeneity.
RESULTS
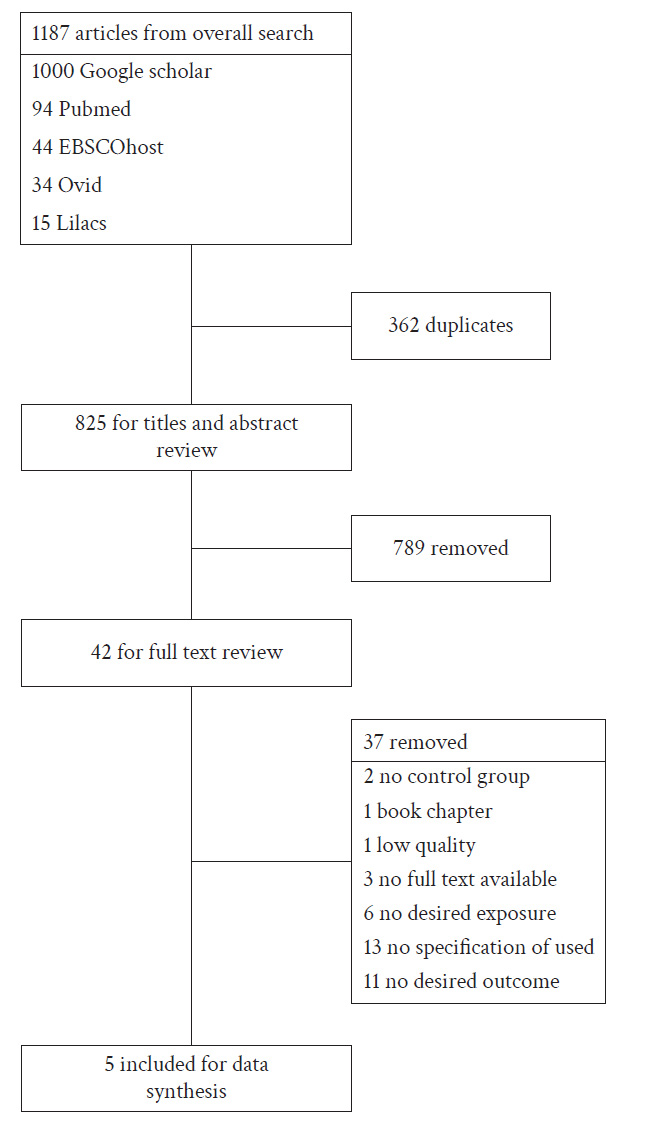
We found a total of 1,187 articles published up to December 31, 2020. Due to download restrictions imposed by Google Scholar, we were only able to retrieve the first 1,000 of the 17,400 articles identified in this database. Additionally, we found 94 articles in PubMed, 44 in EBSCOhost, 34 in Ovid, and 15 in Lilacs.
After removing duplicates, we evaluated 875 unique titles and abstracts. Of these, 42 articles were selected for full-text review. Ultimately, only 5 articles met all inclusion criteria. Figure 1 depicts the study selection process.
The total number of participants across the five studies was 1,656. None of the studies specifically investigated the association between cocaine-derived drug consumption and negative outcomes in TB treatment as their primary objective.
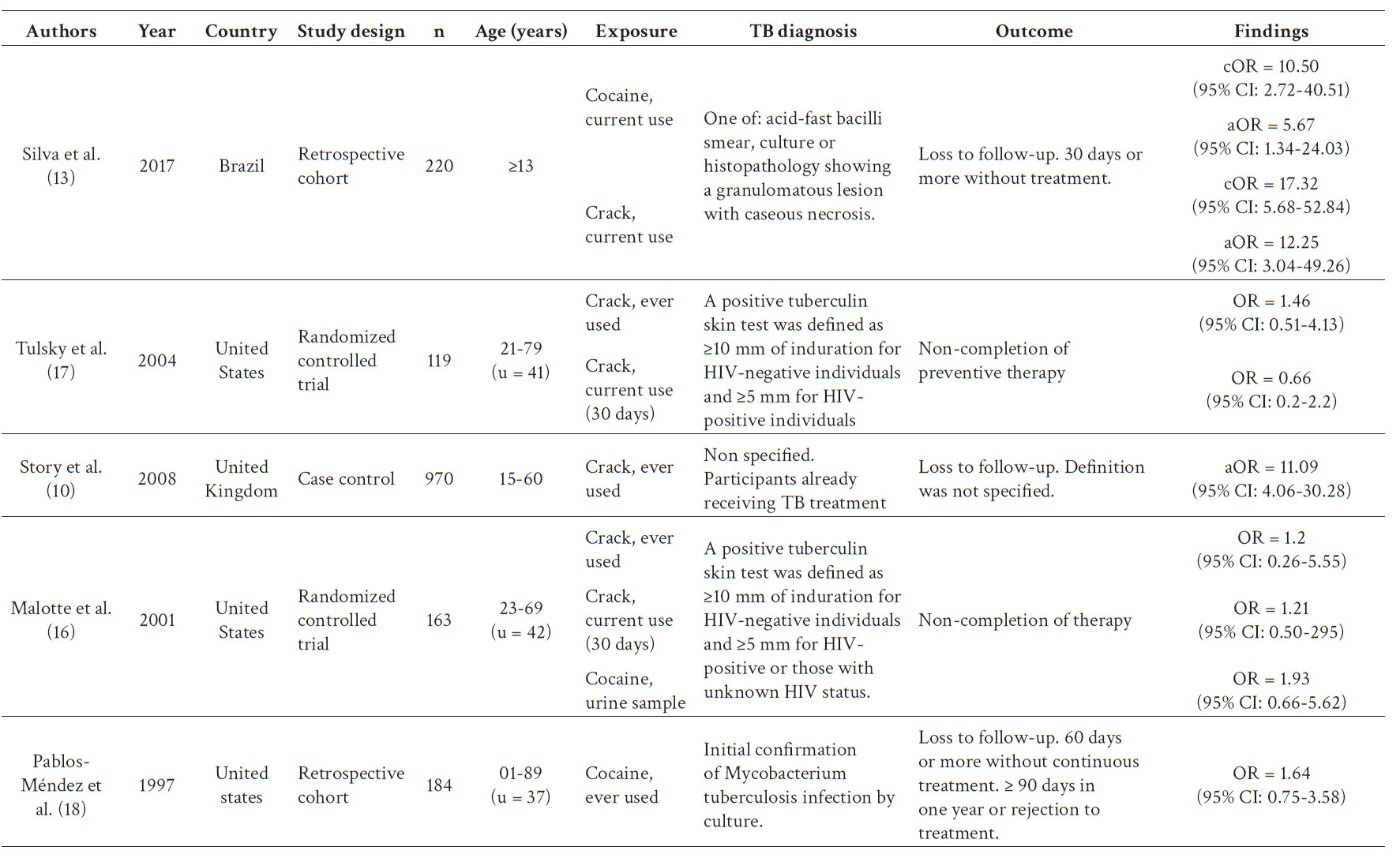
Tulsky et al. 17, Malotte et al. 16, and Pablos-Méndez et al. 18 conducted their investigations in the United States, while Story et al. 10 and Silva et al. 13 conducted theirs in the United Kingdom and Brazil, respectively. Two studies (13, 18) were cohorts, two (16, 17) were clinical trials, and one was a case-control study 10. All studies defined the exposure as the use of cocaine (cocaine hydrochloride or crack), either as the only drug or as the "hardest drug". However, detailed consumption frequency or quantity, and cocaine base paste or local variants were not reported.
Two studies (16, 17) included patients with latent TB, while the other three (10, 13, 18) included patients with active TB. Latent TB was defined as a tuberculin test ≥10 mm in HIV-negative patients and ≥5 mm in HIV-positive patients. Active TB was diagnosed using smear microscopy, culture, or histopathology. The main characteristics of the studies that were considered for data synthesis can be found in Table 2.
The main aim of Silva et al. 13 was to evaluate the risk factors of loss to follow up in TB treatment. They developed a cohort of 220 patients who were categorized into four groups based on their drug consumption characteristics: never used drugs, former illicit drug users, current users of cocaine as the 'hardest drug,' and current users of crack cocaine as the 'hardest drug.'. Patients who didn’t receive treatment for at least 30 consecutive days without medical authorization were considered into the loss to follow up group. Authors notified that the reduction from 60 (WHO definition) to 30 days was arranged by the Brazilian Health Ministry to avoid permanent loss of patients. The bivariate analysis showed that substance use was associated to loss to follow up with a cOR = 10.50 (95% CI: 2.72-40.51) for current cocaine users as hardest drug and a cOR = 4.12 (95% CI: 1.11-15.20) for current crack users as hardest drugs.
Tulsky et al. 17 aimed to establish a positive association between monetary incentives and preventive tuberculosis treatment in homeless or marginally housed people. They did a randomized clinical trial that included 119 patients. Participants without X-ray abnormalities and normal physical findings started isoniazid therapy and were randomized into two groups of intervention. The association between no completion of treatment with sometime in life and actual (last 30 days) crack consumption was non-significant (OR = 1.46; 95% CI: 0.51-4.13 and OR = 0.66; 95% CI: 0.2-2.2, respectively).
Malotte et al. 16 performed a randomized clinical trial including 163 current or former IV drug or crack users. Their objective was to analyze the independent and combined effects between monetary incentives or directly observed therapy in the percentage of patients that completed treatment. Based on the data reported in the paper we estimated that there was no evidence of an association between the sometime-in-life consumers and no completion of treatment (OR = 1.2; 95% CI: 0.68-2.13). Those who reported just crack or crack plus IV drug consumption in the last 30 days obtained a no significant association with no completion of treatment (OR = 1.58; 95% CI: 0.94-2.67) as well as those who got a positive urine examen for cocaine and cocaine plus other drugs (OR = 1.93; 95% CI: 0.66-5.62).
Story et al. 10 performed a case control study that included 970 patients with pulmonary TB currently receiving treatment. They aimed to find the relationship between cocaine consumption and positive sputum samples in pulmonary TBC. The authors do not establish definitions for “loss to follow up”, however they reported the number of patients exposed and not exposed with this outcome. Based upon the information in their study we estimated the association between crack consumption and loss to follow up as an OR = 11.09 (95% CI: 4.06-30.28).
Pablos-Méndez et al. 18 analyzed a retrospective cohort of 184 patients with pulmonary and extrapulmonary TB diagnosed by 1991 and followed up until 1994 in New York. Their objective was to identify predictors and consequences of no adherence to treatment (defined as medical appointment loss for at least 2 consecutive months or 3 or more months for a year). Moreover, they considered non-adherent patients who reject the treatment from the beginning. The OR for cocaine use was 1.64 (95% CI: 0.75-3.58) for non-adherence to the treatment. We considered non-adherence as loss to follow up due to the similarity of the definition the authors used to the one established by the WHO.
Three studies 10,13,18 defined its outcomes as loss to follow up and another two (16, 17) as no completion of treatment. These outcomes will be referred together hereinafter as unfavorable outcomes in TB treatment. No study used death or treatment failure as independent variables. However, we considered the “non completion of treatment” outcome in our analysis because it is an unfavorable outcome in TB treatment.
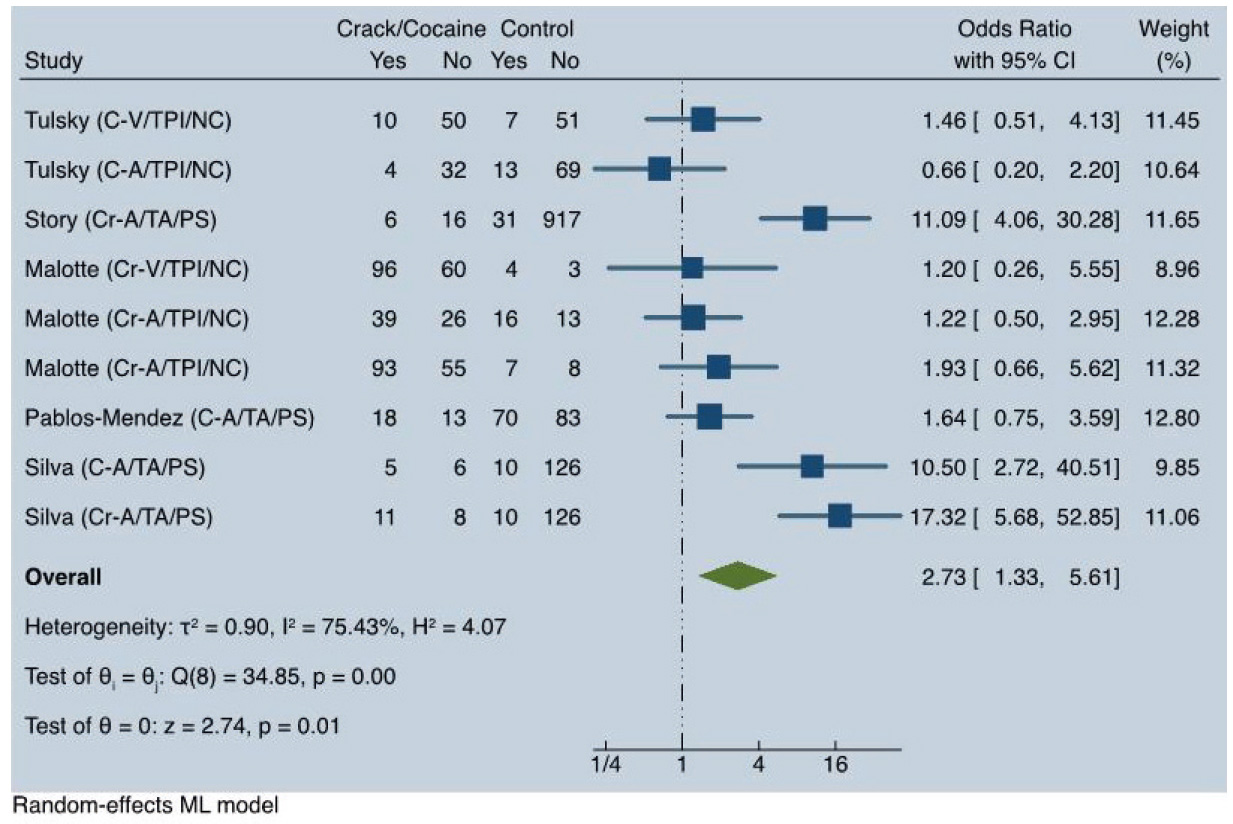
Regarding the loss to follow up and non-completion of treatment, we found a significant statistical association between crack and cocaine hydrochloride use and unfavorable outcomes in TB treatment (OR = 2.73; 95% CI: 1.33-6.61), with very high heterogeneity (I2 = 75.43%). See Figure 2.
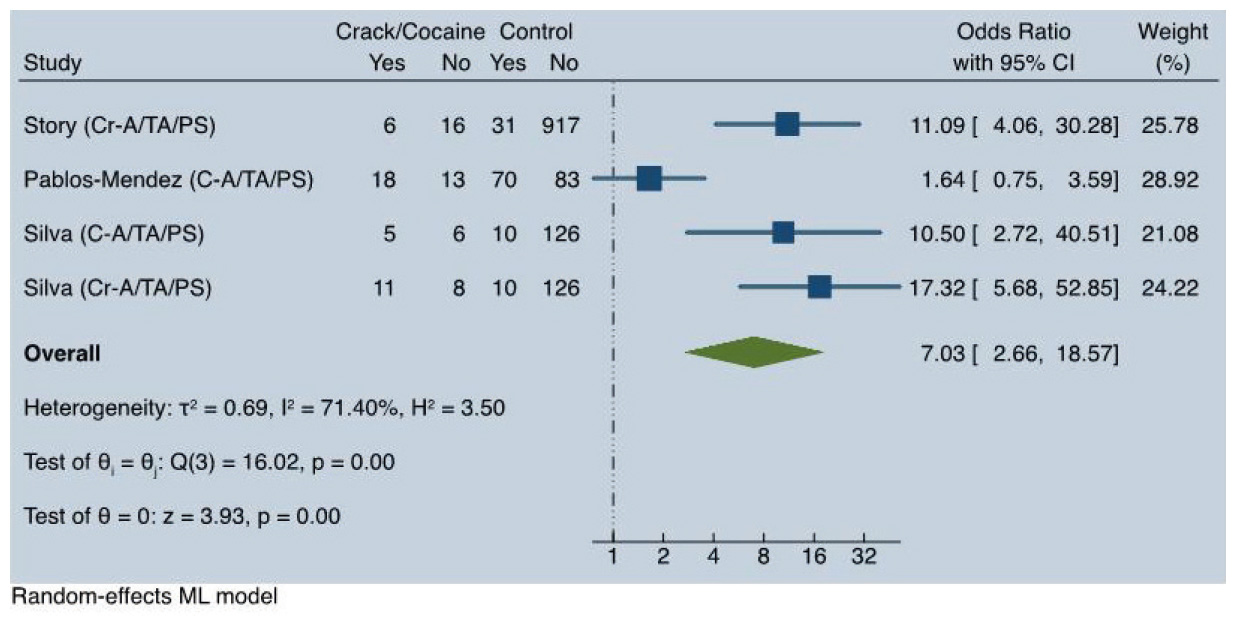
In the subgroup of users of crack or cocaine who received preventive therapy with isoniazid for latent TB, we did not find a significant association with non-completion of treatment (OR = 1.26; 95% CI: 0.77-2.05; I2 = 0%). In the subgroup of patients with active TB, there was a strong association with loss to follow-up (OR = 7.03; 95% CI: 2.66-18.57), although with high heterogeneity (I2 = 71.40%). See Figure 3.
Due to the discordant findings of the Pablos-Méndez et al. 18 study within this subgroup, we conducted a secondary analysis excluding this study. This analysis revealed a strong significant association with no heterogeneity (OR=12.76; 95% CI: 6.64-24.52; I2(0%).
In patients who had consumed these drugs at some point in their lives, there was no significant association with unfavorable outcomes (OR = 1.37; 95% CI: 0.58-3.24; I2(0%).
Current and sometime-in-life crack consumption, when considered together, were associated with unfavorable outcomes in latent or active TB treatment (OR = 3.4; 95% CI: 1.07-5.68; I2 = 76.3%). Current cocaine consumption was significantly associated with loss to follow-up in active TB treatment (OR = 3.48; 95% CI: 1.01-12.01; I2 = 61.8%), as was current crack consumption (OR = 13.54; 95% CI: 6.42-28.56; I2 = 0%). However, current crack consumption did not show a significant association with non-completion of latent TB treatment (OR = 1.21; 95% CI: 0.67-2.18; I2=0%).
All studies were individually evaluated for their quality of evidence using the criteria described previously. Four studies (13, 16-18) received a poor-quality score due to deficiencies in the selection and comparability sections. The exposed and non-exposed groups to cocaine and crack were not described individually, making it impossible to assess if they were significantly different. These studies were not designed according to our PICO question and did not address the same exposures and outcomes. In contrast, the Story et al. 10 study obtained a good quality score.
DISCUSSION
The present study aimed to synthesize the available information on the association between illegal cocaine-related drug consumption and negative outcomes in TB treatment, specifically death, loss to follow-up, or treatment failure, considered individually or together. No studies were found to have been designed to answer our research question as their main analyses. Our results are based on secondary analyses of loss to follow-up and non-completion of treatment outcomes.
Our results suggest that there is an association between cocaine or crack (sometime-in-life or current) and loss to follow-up or non-completion of TB treatment (OR = 2.73). This association tends to be strong and statistically significant in people undergoing active TB treatment (OR = 7.03) but is non-significant in those with latent TB treatment (OR = 1.26; 95% CI: 0.77-2.5). We considered cocaine hydrochloride and crack as exposures likely to be analyzed together because they produce similar effects, being related to the same base substance 19.
We did not find comparative studies between active and latent TB regarding adherence or negative outcomes, only descriptions of these outcomes considered individually. The percentage of patients with latent TB who complete their treatment varies widely within subgroups: from 22% (6-43%) in prisoners to 82% (66-94%) in patients with HIV. Among patients with active TB, the percentage of success in treatment (healed and treatment completed) is approximately 85% in patients with no resistant TB and 57% in MDR TB 2. The WHO has identified the therapeutic regime as a factor associated with TB treatment adherence 20. Due to the greater complexity of active TB treatment compared to latent TB, we suggest that the difference found in our review may be explained by this factor. Moreover, the two studies included that evaluated latent TB treatment outcomes had monetary and non-monetary incentives (16, 17). All participants in the Tulsky et al. 17 and Malotte et al. 16 studies received directly observed therapy (DOT), a WHO-driven strategy. Although a Cochrane publication shows no significant difference in cure or completion of treatment between conventional DOT and self-administered treatment, some studies suggest a significant difference, especially in community-based strategies 21-23. Additionally, monetary incentives have been proven to favor positive outcomes in TB treatment 24. Both DOT and monetary incentives could modify the outcomes, and as long as these factors could not be controlled in the statistical analysis, it is possible that we have underestimated the relationship between crack or cocaine consumption and non-completion of latent TB treatment.
When analyzing all included studies and the active TB subgroup, we observed very high heterogeneity (I2 = 75.4%) and high heterogeneity (I2 = 71.4%), respectively. As shown in Figure 3, when only the results from Story et al. 10, Pablos-Méndez et al. 18, and Silva et al. 13 were included, the data were heavily influenced by the results of Story et al. 10 and Silva et al. 13, which accounted for 71% of the weight. Both studies reported high point estimates of ORs, ranging from 10.50 to 17.32, whereas Pablos-Méndez et al. 18 reported no statistically significant association (OR = 1.64; 95% CI: 0.75-3.58).
Pablos-Méndez et al. 18 conducted patient monitoring between 1991 and 1994, during which a significant intervention was implemented in New York. Between 1992 and 1995, the Correctional Services Department collaborated with the Conditional Freedom Division and the Health Department to diagnose and treat latent and active TB in 70 correctional facilities, involving 69,000 patients. Active TB patients were isolated for at least two weeks until laboratory confirmation of non-contagiousness was achieved. Additionally, treatment was delivered through the DOT strategy, which was mandatory under New York public health law. This extensive intervention during patient monitoring could explain the discordant outcomes in TB treatment observed in this study.
In contrast, the definition of loss to follow-up used by Silva et al. 13 was 30 or more days without treatment, whereas Story et al. 10 employed a more flexible definition: 60 or more continuous days without treatment, 90 days or more without treatment in one year, or outright rejection of treatment. These differences in outcome definitions likely contributed to the variation in findings. Furthermore, the classification of cocaine-based drug use also varied. Pablos-Méndez et al. 18 used “ever use”, similar to Silva et al. 13 in Brazil, while Story et al. 10 focused on “current use”. These differences in exposure definitions may further explain the disparities in results.
It was not possible to conduct a subgroup analysis by TB location (pulmonary/extrapulmonary) or resistance due to the lack of data in the included studies to calculate associations between these subgroups.
Furthermore, current crack consumption showed a stronger association than current cocaine use (OR = 14.54; 95% CI: 6.42-28.56 vs. OR = 3.48; 95% CI: 1.01-12.01) with loss to follow-up outcomes in patients with active TB. Crack consumption is known to be more related to psychosocial problems such as delinquent behavior, psychotic symptoms, and violent acts and thoughts, as well as with an intense dependence on consumption compared to cocaine hydrochloride. Crack has a higher bioavailability, stronger post-consumption dysphoria, an easier method of administration and a more accessible price 19. These factors could explain why crack consumers have worse outcomes in anti-TB treatment.
Substance abuse is more common among males, but the role of male gender in TB treatment outcomes is still unclear. While some authors do not find a significant association between male gender and negative outcomes in TB treatment 25-28, other studies establish it as an independent risk factor for loss to follow-up in TB treatment (8, 29). Various studies have reported a high prevalence of a low education level among drug consumers (10, 27, 28). Additionally, there is evidence of significant association between education level and adherence or treatment failure in TB treatment. Persons with lower education degree have showed higher risk of negative treatment outcomes (30, 31), suggesting that education level plays a modifying role in the results. Evidence supports that cocaine-derived users are often polysubstance consumers, frequently including alcohol 32. However, no study specifies the percentage of patients exposed to other substances, although Silva et al. 13 found an association between high-risk alcoholism (CAGE Scale 3-4) and loss to follow-up (OR = 6.1; CI95%: 2.86-13.26). Other authors have also reported an association between alcohol consumption and negative outcomes in TB treatment 32. Moreover, low economic income, homelessness, and unemployment have been established as risk factors for negative outcomes 32.
One important limitation of our study is that, as previously stated, no study specifically designed to address our research question was available in the reviewed literature. This limitation suggests that the results and conclusions may be subject to selection and information bias, which might not have been fully accounted for. For instance, the primary aim of the original research by Story et al. 10 was to investigate the association between crack use and smear-positive TB. The variable related to loss to follow-up was included as a covariate rather than a primary outcome and was not consistently utilized across studies.
Another important limitation of our study is that none of the final articles evaluated their loss to follow-up outcomes strictly according to the WHO's definition. Silva et al. 13 reduced the required number of days due to governmental reasons; Story et al. 10 did not define loss to follow-up, Pablos-Méndez et al. 18 used a looser definition, and the others reported the percentage of patients who completed the treatment. This heterogeneity in the definition of loss to follow-up may be explained by temporality; some studies were conducted before the current international definitions presented by the WHO in 2013 15
Another important limitation of our study is the inability to extract key variables from all studies, which are essential for controlling confounding bias. For instance, co-occurring use of alcohol and other legal or illegal substances is associated with both an increased likelihood of cocaine-based drug use and a higher risk of negative TB outcomes. Additional confounding factors that could influence the association between cocaine-based drug use and adverse TB treatment outcomes include male sex, level of education, marital status, employment status, and characteristics of healthcare facilities, particularly their capacity to manage withdrawal symptoms.
Additionally, we could not access all the articles available in Google Scholar due to the download limit established by the browser, dismissing 16,400 articles and retrieving only the first 1,000, which were more similar to our search terms according to the tool. There were other databases that were not consulted (e.g., EMBASE) because we did not have access to them. However, we conducted a supplementary search of clinical trials in the Cochrane database and did not find any additional studies. Finally, we included only articles written in English and Spanish, which can imply a publication bias. This might be of relevance, since we might have not included data from countries that are less likely to report their results in English or Spanish, but are key to understand TB epidemiology, such as the eastern Europe countries and Russia Federation, some countries in Africa and Asia.
The meta-analysis showed high heterogeneity between studies (I2=75.43%). This may be explained by differences in outcome definitions, the fact that some studies evaluated loss to follow-up only in active TB patients while others evaluated non-completion of treatment only in latent TB patients, and the lack of information on drug consumption frequency and quantity in some studies. This high heterogeneity is probably the result of the influence of the data extracted by Silva et al. 13 and Story et al. 10. Both studies were performed in patients with active TB patients, and one of them in a developing country, which might explain in part the high heterogeneity found. We advise to interpret the results of the synthesis taking this into consideration.
During the systematic review, we found 14 articles that did not specify the illegal drug used. We requested this information via email, but only six authors responded, and none had the required data. We suggest that future investigators of the risk factors for negative outcomes in TB treatment specify the type of substance used and, if possible, the characteristics of drug use (frequency and quantity). Also, it will be important to include information from regions of high incidence of TB.
The clinical implications of our study highlight the use of illegal drugs, particularly cocaine-based substances, as significant risk factors for adverse outcomes in TB treatment. The strength and clinical relevance of this association may vary considerably depending on the target population, as indicated by the high heterogeneity observed in our analysis. Consequently, the detection and clinical management of cocaine-based drug use during TB treatment should be key targets for interventions aimed at improving TB outcomes, both as a public health strategy and at the individual level.
CONCLUSIONS
The present study provides evidence supporting an association between crack/cocaine consumption and loss to follow-up or non-completion of treatment for tuberculosis. This association is apparent in active TB treatment but not in latent TB treatment. Furthermore, the association appears to be stronger among crack users.
However, it was not possible to conduct a subgroup analysis by TB location (pulmonary/extrapulmonary) or resistance due to the limitations of the available data. Additionally, no studies were found that directly evaluated this association. Therefore, we recommend continuing the search for evidence that directly assesses the relationship between cocaine-derived drug consumption and negative outcomes in TB treatment.