Introduction
The widespread freshwater cyclopoid copepod genus Mesocyclops Sars, 1914 can be found in a wide variety of freshwater habitats, including temporal and permanent pods, lakes, rivers, swamps (Hołyńska et al. 2003, Alekseev et al. 2013, Papa and Hołyńska 2013, Fuentes- Reinés et al. 2013), and even coastal hiposaline ponds (Suárez-Morales et al. 1999). It is considered as a cosmopolitan group with greater affinity for tropical areas, especially in eutrophic water bodies (Luong et al. 2020). This genus is one of the most speciose in the subfamily Cyclopinae; with nearly 80 valid species known (Papa & Hołyńska 2013, Tran & Hołyńska 2015), only 21 have been reported from the Neotropical region (Gutiérrez- Aguirre et al. 2006, Suárez-Morales et al. 2020). Of these, only six have been hitherto recorded from Colombia: Mesocyclops aspericornis (Daday, 1906), M. brasilianus Kiefer, 1933, M. longisetus (Thiébaud, 1912), M. meridianus (Kiefer, 1926), M. reidae Petkovski, 1986, and M. ellipticus Kiefer, 1936.
Mesocyclops paranaensis, originally reported as M. meridianus and M. ellipticus has been recorded from Paraguay and Brazil respectively (Lowndes 1934, Dussart & Frutos, 1986, Gutiérrez-Aguirre et al. 2006). Mesocyclops paranaensis is restricted to the Neotropical region (Gutiérrez- Aguirre et al. 2006, Suárez-Morales et al. 2020).
The aim of this contribution is to document the first record of M. paranaensis for Colombia, which expands its known distributional range in South America, and also to provide comparative morphological data and illustrations of the Colombian specimens.
Material and method
Biological samples were obtained from a small temporary pond in the northern part of La Guajira-Colombia (11°16'24.67"N,73°07'22.92"W). Qualitative surveys were performed during October and November 2018. Environmental parameters were measured with a WTW 3111 conductivity meter gear. Water samples were obtained using a bucket of 65 L, filtered with a 55μm meshsize plankton net to obtain concentrates of 500 mL that were fixed and preserved in 96% ethanol. In the laboratory, samples were stained with Bengal rose and concentrated to a volume of 50 mL. A Bogorov chamber was used to sort and count copepods, with the aid of a stereomicroscope; copepods were taxonomically examined under a compound optical microscope in a drop of glycerol. Specimens were measured in ventral position, from the anterior end of the cephalothorax to the posterior margin of the caudal ramus and then dissected to examine the taxonomically relevant appendages, which were mounted in semi-permanent slides. The appendages with taxonomic relevance were photographed using a Kodak Easy Share C140 digital camera adapted to a compound microscope at 1000× magnification. Drawings of taxonomically relevant structures were prepared with the aid of a camera lucida mounted on an Olympus BX53 semi-motorized microscope with Differential Interference Contrast optics. The identification of this species followed the keys, illustrations, and descriptions by Dussart & Frutos (1986), Gutiérrez-Aguirre et al. (2006), and Suárez-Morales et al. (2020). Our morphological remarks and complementary description followed Huys and Boxshall (1991). The following abbreviations are used in the text: P1−P6 = first to sixth legs; EXP = exopod; ENP = endopod.
Voucher specimens of M. paranaensis were deposited at the Centro de Colecciones Biológicas held at the Universidad del Magdalena, Colombia (CBUMAG:MEI:0827) where they are available for consultation and/ or further examination.
Results
Order Cyclopoida Burmeister, 1835
Family Cyclopidae Rafinesque, 1815
Subfamily Cyclopinae Rafinesque, 1815
Genus Mesocyclops Sars, 1914
Mesocyclops paranaensis Dussart & Frutos, 1986, p. 312, fig. 46-50
Mesocyclops meridianus in Lowndes (1934: p. 80, 107)
Material examined: 7 adult female specimens collected by one of us (JMF-R) from an ephemeral pond located in La Guajira, northern Colombia (11°24´32,70"N, 73°03´45,75"W) during October and November 2018.
Morphology. Body length of Colombian female specimens = 1064 -1148 μm (n = 7, average length = 1122 μm) (Fig. 2A). Body slender, with robust anteriorly rounded prosome; fifth pedigerous somite lightly pilose. Antennules 17-segmented (Fig. 2B), two distal segments with serrate hyaline membrane (Fig. 2C), distal half of hyaline membrane on last segment with three strong denticles forming deep notch (Figs. 1B, 2C); length ratio of segments 16/17 = 1.03. Antenna composed of basipodite and 3-segmented endopodite (Fig. 3D). Frontal surface of basipodite with several spinule rows (Fig. 1C). Antennary Enp 2 with 7 setae (arrowheads in Fig. 3D), 4 of them long, inserted distolaterally; remaining 3 setae short, slender on medial position (Fig. 3D). Antennary Enp3 with 7 setae, 4 of them thicker than remaining 3 (Fig. 1M). Antennary exopod represented by single strong pinnate seta (arrow, Fig. 3D); exopod insertion point in the frontal surface of coxobasis with spinules.
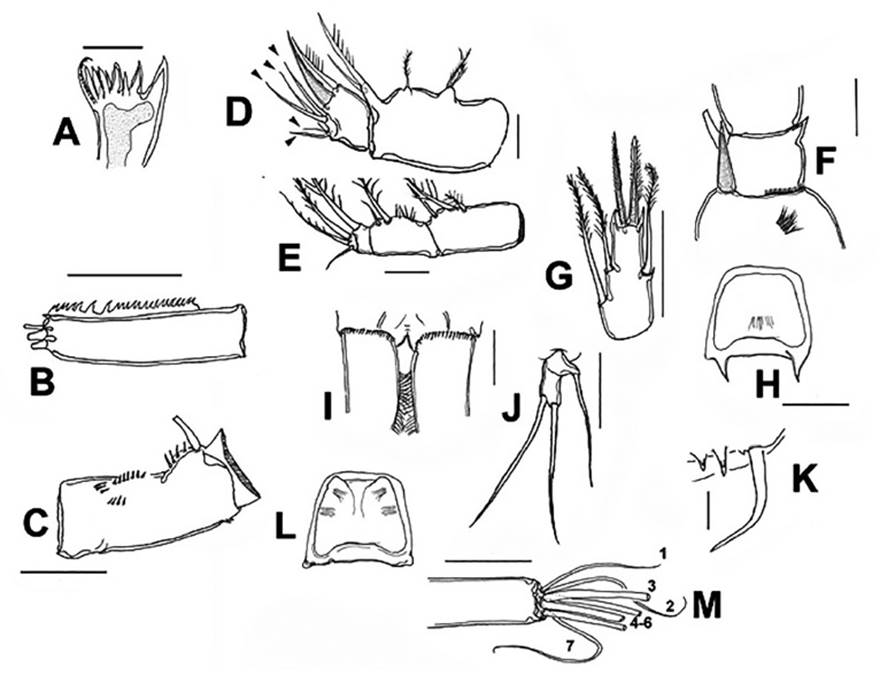
Figure 1 Mesocyclops paranaensisDussart & Frutos, 1986, adult female from ephemeral pond, northern Colombia. A. mandible blade. B. antennule, last segment showing serrate hyaline membrane. C. antennary basipodite, frontal view. D. maxilla with endopodal setae arrowed. E. maxilliped. F. P1 inner basipodal spiniform seta. G. P4 ENP3. H. P4 intercoxal sclerite, caudal view. I. caudal rami and posterior margin of anal somite, dorsal view. J. P5. K. P6. L. P3 intercoxal sclerite. M. distal setae of third antennary endopodal segment. Scale bars: A, J = 25 μm, B, C, G, H, M = 50 μm, F= 25 μm.
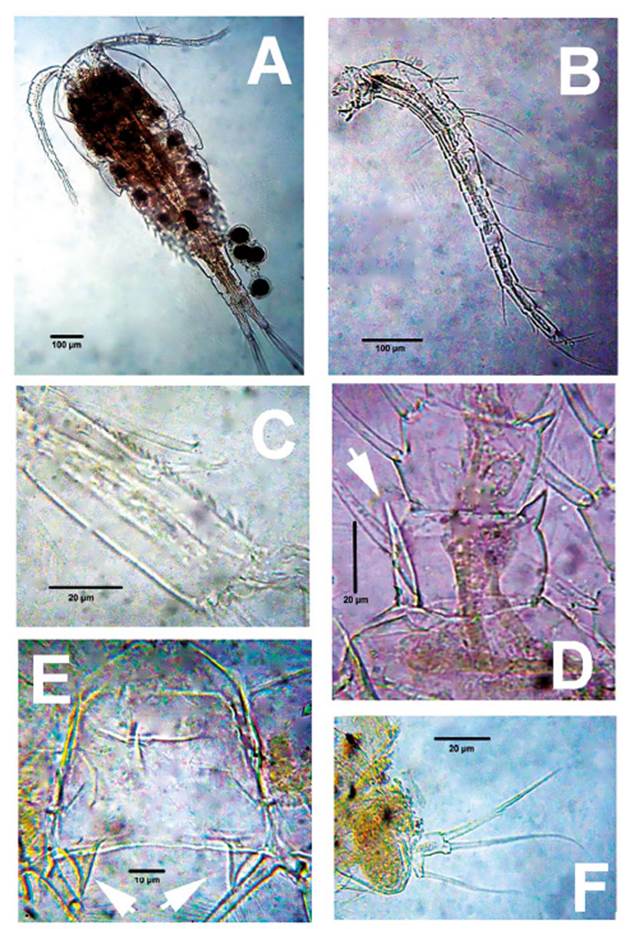
Figure 2 Mesocyclops paranaensisDussart & Frutos, 1986, adult female from ephemeral pond, northern Colombia. A. Habitus, dorsal view. B. antennule. C. same, last segment showing hyaline membrane. D. P1 inner basipodal spine (arrowed). E. P4 intercoxal sclerite, caudal view, showing acute processes (arrowed). F. P5.
Mandible gnathobase with three strong and four slender teeth plus dorsal seta with pinnate inner margin (Fig. 1A); as usual in genus, mandibular palp with 2 long and single short setae.
Urosome 5-segmented, ventral genital double somite smooth, expanded proximally (Fig. 3E), seminal receptacle weakly convex (arrow, Fig. 3C) with narrow lateral arms. Posterior margin of anal somite with continuous row of spinules dorsoventrally (Fig.1I); caudal rami 2.8 times as long as wide, inner margin hirsute (Fig. 1I). Lateralmost terminal caudal seta 3 times as long as lateral caudal seta, lateral median terminal caudal seta 1.4 times as long as medialmost terminal caudal seta, medial median terminal caudal seta 1.2 times as long as urosome, dorsal caudal seta as long as lateralmost terminal caudal seta.
Maxilla (Fig. 1D) and maxilliped (Fig. 1E) structure and armature typical of genus (Suárez-Morales & Gutiérrez- Aguirre 2001).
P1-P4 EXP and ENP 3-segmented. Inner basipodal margin of P1 with short, strong spine barely reaching beyond distal margin of first endopodal segment (Figs. 1F, 2D, 3A), frontal surface of basipodite ornamented with long spinules (Fig. 1F), intercoxal sclerite of P1-P2 caudally smooth; caudal surface of P3, P4 sparsely pilose (Fig 1H, 1L). Intercoxal sclerite of P4 with pair of large, acute projections (Figs. 1H, 2E, 3B). P4ENP3 2.25 times as long as wide, terminal spines subequal (Fig. 1G, 3B). Pediger 5 laterally pilose, P5 typical of genus, with relatively long proximal seta (Figs. 1J, 2F). P6 with two short spiniform elements plus one long seta (Fig. 1K).
Discussion
The morphology of the seven adult female specimens from La Guajira, Colombia agrees with previousdescriptions and illustrations of the species (Dussart& Frutos 1986, Gutiérrez-Aguirre et al. 2006, Suárez-Morales et al. 2020).
Mesocyclops paranaensis can be separated from itsclosest congeners by a unique combination of characters including: 1) leg 4 intercoxal sclerite with two lar-ge, acute projections, 2) P3, P4 intercoxal sclerite caudally slightly pilose 3) seminal receptacle with narrowlateral arms and anterior margin weakly convex, 4) antennary endopodite-2 with seven setae and insertion ofantennary exopodal seta with spinules, 5) frontal surfaces of P1 basipodite ornamented with long spinules, 6)posterior margin of female anal somite with continuousrow of spinules, and 7) inner margin of caudal ramushirsute. These distinctive traits are present in our Colombian specimens.
A few subtle differences were observed in our Colombian specimens: 1) the inner distal spine of P4ENP3 is slightly longer than the outer spine (Fig. 1G) vs. equally long spines in the Argentinian population (Dussart & Frutos 1986, fig. 46); 2) with a size range of 1064 - 1148 μm, our female specimens are noticeably smaller than those examined by Dussart and Frutos (1986) (1700 μm) and Hołyńska et al (2003) (1200 - 1700 μm) from Argentina and by Lowndes (1934) (1244 - 1550μm) from Paraguay; 3) the P4ENP3 is 2.25 times as long as wide in our Colombian specimens (Figs. 1G, 3B) vs. 2.2 - 2.5 in the Argentinian populations (Dussart & Frutos 1986, fig. 46; Hołyńska et al. 2003, fig. 30A). Also, the lateral arms of the seminal receptacle are straight in the Argentinian population (Dussart & Frutos 1986, Fig. 49) and slightly curved in the Colombian specimens (Fig. 3C). In addition, this species appears to be uncommon within its distribution range and only a few specimens have been taxonomically examined (Hołyńska et al. 2003); until sufficient comparative information is available, it is premature to suggest that the Colombian population could represent a distinct species. Instead, it is suggested that sampling efforts should be increased in the species distributional range to examine its intraspecific variability in more detail and thus define its taxonomic status within the genus.
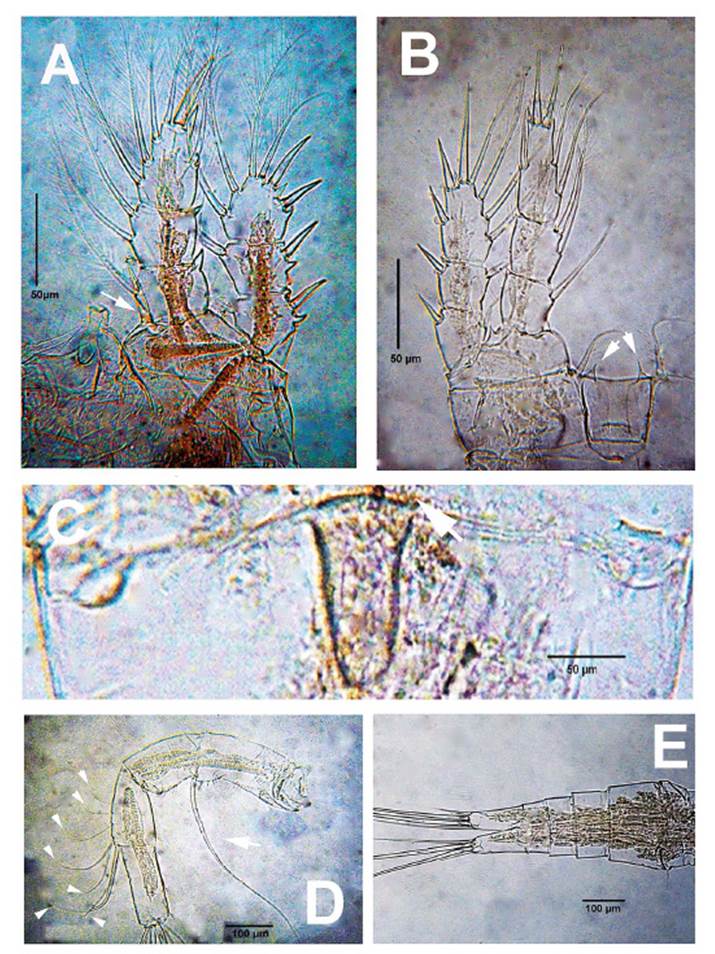
Figure 3 Mesocyclops paranaensisDussart & Frutos, 1986, adult female from ephemeral pond, northern Colombia. A. P1, anterior view showing inner basipodal spine (arrowed). B. P4 anterior view showing intercoxal sclerite with acute processes (arrowed). C. genital field, ventral view showing weakly convex anterior margin of seminal receptacle (arrow). D. antenna showing exopodal seta (arrow) and Exp2 setae (arrowheads). E. urosome, ventral view.
The genus Mesocyclops was divided by Hołyńska (2006) into seven clades. Mesocyclops paranaensis was included in the annulatus-clade which contains at least three other Neotropical species (i.e., M. intermediusPesce, 1985; M. ellipticus Kiefer, 1936; M. annulatus (Wierzejski, 1892)), and one Afrotropical (M. tenuisaccus (G.O. Sars, 1927)).
In the Neotropical region, M. paranaensis can be confused with M. intermedius and M. annulatus but they can be distinguished by the following characters: 1) the ornamentation of the posterior margin of the anal somite of M. paranaensis and M annulatus consists of a dorsoventrally continuous spinule row (Gutiérrez-Aguirre et al. 2006, fig. 8F, present data, Fig. 2I, Hołyńska et al. 2003, fig. 26H) whereas a discontinuous pattern is present in M. intermedius (Gutiérrez-Aguirre et al., 2006, fig. 8E); 2) the inner margin of caudal rami is hirsute in both M. paranaensis and M. annulatus (Gutiérrez-Aguirre et al. 2006, fig. 8F, present data, Fig. 1I; Holyńska et al. 2003, figs. 26G) vs. a few scattered hair-like elements found in M. intermedius (Gutiérrez-Aguirre et al. 2006, fig. 8E; Suárez-Morales et al. 2020, fig. 21.25 U); 3) the antennary Enp2 of both M. paranaensis and M. intermedius are armed with 7 setae (Hołyńska et al. 2003; present data, Fig. 3D) vs. 9 such setae in M. annulatus (Hołyńska et al. 2003); 4) inner spine on P4EXP3 almost as long as the outer spine in M. paranaensis (Dussart & Frutos 1986, fig. 46; Holyńska et al. 2003, fig 30H; present data, fig. 3B) and M. intermedius (Pesce 1985, fig. 64) whereas in M. annulatus they are unequal in length (Hołyńska et al. 2003, figs. 27J); 5); frontal surface of P1 basipodite ornamented with long spinules in M. paranaensis (Hołyńska et al. 2003, fig. 30G; present data, Fig. 1F) and M. intermedius (Hołyńska et al. 2003, fig. 32G) whereas in M. annulatus the long spinules are absent (Hołyńska et al. 2003, fig. 27A); 6) length ratio of seta on proximal segment/ distal seta on second segment on P5 less than 1 in M. paranaensis (Dussart & Frutos 1986, fig. 47; present data, Figs. 1J, 2F,) and M. annulatus (Hołyńska et al. 2003, fig. 26C) whereas in M. intermedius greater than 1(Pesce 1985, fig. 60); 7) length/width ratio of caudal ramus is smaller in M. paranaensis (about 2.8 - 4.2, Hołyńska et al. 2003, fig. 30A; present data, fig. 3E), and M. intermedius (3.0, Pesce 1985, fig. 60; Hołyńska et al. 2003, fig. 32C) than in M. annulatus ( 5.0 - 5.5, Hołyńska et al. 2003, figs. 26H); and 8) M. intermedius is a smaller (520 - 610 μm) species (Pesce 1985) than M. paranaensis (1064 - 1770 μm, Dussart & Frutos 1986; Lowndes 1934; Hołyńska et al. 2003; present data) and M. annulatus (1300 - 2000 μm Hołyńska et al. 2003).
In the surveyed area, M. paranaensis was found associated to the aquatic vegetation (water temperature = 31.4 °C, conductivity 255 μS/cm, pH 7.68). The presence of M. paranaensis in adjacent areas seems very likely, so a wider distribution in South America might be expected. The diversity and distribution of this genus in the Neotropical region deserve further research.












 uBio
uBio 


