1. Introduction
The continuous need to increase agricultural productivity requires soil conservation, pest control, selection of productive plants, and minimizing environmental impacts while improving the nutritional quality of crops (Hemathilake & Gunathilake, 2022). Agricultural inputs, such as fertilizers, fungicides, and bactericides, play a key role in enhancing plant characteristics and soil properties by improving nutrient availability and understanding soil microbiota and plant-insect relationships (Miller et al., 2022).
The use of metal chelates in fertilizers and bactericidal products is an area of interest due to their ability to provide bioactive metal ions efficiently while minimizing toxicity and environmental impacts (Paterson et al., 2022; Sharma et al., 2022). Copper (Cu II) is an essential micronutrient for plants but has a narrow toxicity range. It is absorbed as Cu(II) and Cu(I) cations, predominantly complexed by soil organic matter, which reduces toxicity and increases availability for plant roots (Eduah et al., 2024). Soil pH, organic matter, and clay content influence copper availability; acidic soils favor the free form of Cu(II), which is the most reactive and phytotoxic form (Wan et al., 2024). Organic matter mitigates toxicity by forming stable complexes with copper, enhancing plant absorption.
Chelators such as aminopolycarboxylates (APCAs) are widely used to stabilize metal ions, including EDTA, DTPA, and DOTA, among others (Stanojević et al., 2021). APCAs form highly stable, water-soluble complexes with metals, improving their availability and transport in plants across a wide pH range (Flora & Pachauri, 2010; Pokethitiyook & Poolpak, 2016). The stability of these complexes follows the order: NTA < EGTA < DCTA < EDTA < PDTA < DTPA < DOTA (Martins et al., 2005).
Parsley manioc (Arracacia xanthorrhiza Bancroft), also known as mandioquinha or batata-baroa, is a carbohydrate-rich, nutrient-dense crop primarily cultivated in high-altitude regions of South America and Brazil (Sediyama et al., 2005; Madeira et al., 2017). However, its production is often affected by soft rot caused by Erwinia chrysanthemi, a gram-negative, facultative anaerobic bacterium. The disease manifests through wilting, tissue rot, and foul odor, exacerbated under high temperature and humidity conditions. Erwinia utilizes quorum-sensing, a bacterial communication mechanism, where short-chain acyl-homoserine lactones trigger virulence once a critical bacterial density is reached, reducing the plant’s defenses and intensifying the disease (He et al., 2024; Pilgrim, 2024).
To manage soft rot, bactericidal treatments that are non-phytotoxic and safe for consumers are required. This study aimed to synthesize and characterize Cu(II)-PDTA complexes using sodium and carbonate pathways and evaluate their in vitro bactericidal activity against Erwinia chrysanthemi, the main cause of soft rot in parsley manioc.
2. Methodology
2.1. Synthesis of metal chelates
The PDTA chelator (Trilon F) was supplied by BASF S/A with the binder in both the sodium salt and acid forms and utilized for the chemical synthesis by two synthetic routes of the aminopolicarboxylate complex, Na2[Cu (II)(PDTA)] via sodium and [Cu (II)(PDTA)] via carbonate. Both synthetic routes were carried as described elsewhere (Skoog et al., 2004; Carvalho et al., 2005). The use of chelator / binder in the form of sodium (direct pathway complexation) involved the formation of the [Cu (II)(PDTA)] complex and the secondary product, Na2SO4. On the other hand, carbonate synthesis with (insoluble) PDTA acid involved the reaction of hot complexation of the metal with the suspended carbonate and elimination of carbon dioxide.
2.2. Carbonate chelate synthesis
To prepare the complex at a stoichiometric ratio of 1:1, 4.0 g of copper pentahydrate (CuSO4∙5H2O 0.010 mol) (Sigma-Aldrich / VETEC), 98% pure, was weighed and added to a saturated sodium hydrogen carbonate solution (NaHCO3) stirring slowly until all bicarbonate reacts. The precipitate was then washed with distilled water using centrifuge. Qualitative analyzes with barium chloride (BaCl2) were performed at each wash step to verify the presence of interfering ions. After washing, the copper carbonate was taken into a water bath to obtain the copper carbonate (CuCO3) in solid form, with green-water color. Continuing the synthesis, 1.3 g of CuCO3 (0.010 mol) partially dissolved in water under heating and stirring was added before 3.3 g of PDTA (0.010 mol) binder in acidic form H4-PDTA (BASF S/A). The resulting reaction solution showed intense royal blue color. A simple filtration process was then carried out and the metal complex solution was dried in a water bath to obtain the solid metal complex [Cu (II) (PDTA)].
The reaction steps for the synthesis of the Cu (II)(PDTA)]-2 ion complex via carbonate are represented by the Equations 1, 2 and 3:
CuSO4∙5H2O (aq) + 2 NaHCO3 (aq) → CuCO3(s) + Na2SO4 (aq) + 6 H2O (l) + CO2 (g) (1)
H4PDTA (s) + CuCO3 (aq) → [Cu (II) (PDTA)] (aq) + 2 H2O (l) + ½ CO2 (g) (2)
[Cu(II)(PDTA)]-2 (aq) → [Cu (II) (PDTA)]-2 (s)(3)
2.3. Synthesis of Na2 [Cu (II) (PDTA)] and Na2SO4 chelate via sodium salt
Synthesis of the [Cu (II) (PDTA)] complex via sodium salt was performed using PDTA sodium salt (Na4PDTA) (BASF S/A) with 99% purity. The synthesis of the complex was performed in the 1:1 molar base stoichiometric ratio from the metal salt CuSO4∙5H2O (2.7 g, 0.010 mol) and PDTA salt (4.0 g, 0.10 mol). Acid neutralization was carried out with sodium hydroxide (NaOH) under mild heating to accelerate dissolution and product formation. After mixing, the pH was adjusted to the range of 7.5 to 8.0 and the binder Na4PDTA was slowly added over the CuSO4∙5H2O solution under constant stirring. The complexation reaction occurred without precipitation and with the formation of the salt Na2SO4. The reaction solution was taken to a water bath to evaporate water in order to obtain the reaction products Na2[Cu (II) (PDTA)] and Na2SO4 in solid form. The reaction steps for the synthesis of the Na2[Cu (II) (PDTA)] ion complex via sodium is indicated by the Equations 4 and 5:
CuSO4∙5H2O (aq) + Na4PDTA (aq) → Na2[Cu(PDTA)] (aq) + Na2SO4 (aq) + 5 H2O (4)
Na2[Cu (II)(PDTA)](aq) + Na2SO4 (aq)→Na2[Cu (II)(PDTA)] (s) + Na2SO4 (s)(5)
2.4. Characterization of the metal complex (via carbonate) and complex mixture (via sodium)
The [Cu (II) (PDTA)] complexes were synthesized in a 1:1 molar ratio and characterized by UV-Vis Electron Absorption Spectroscopy and Infrared (IR) Absorption Spectroscopy, in addition to the biological activity performed by diffusion antibiogram.
2.5. UV-Vis molecular absorption spectroscopy
Spectroscopy in the UV-Vis region is based on the absorption of radiation in the range of 190 to 800 nm by inorganic and organic molecules. Molecular absorption is the result of the interaction between photons and electrons of the atoms participating in the bond. The most common atoms of ligands are oxygen, sulfur, nitrogen or chlorine. The bond (with metal binder) has displacements at maximum absorption or along the radiated wavelength (Martell & Smith, 2001). The UV-Vis was used for the characterization of metal complexes by comparison of absorption spectra and analysis of the displacements of PDTA bands, metal and formed complex. Absorption spectra were obtained on a Cary Eclipse Spectrofluorimeter (Varian) with a 200 to 500 nm scan. Analyzes were carried out at a concentration of 5 10-4 mol L-1, and the samples were diluted in acetonitrile (solvent) using an ultrasonic bath.
2.6. Infrared (IR) absorption spectroscopy
Infrared (IR) spectroscopy is a technique used for covalently bonded compounds. The IR corresponds to the range encompassing vibrational stretching and bending frequencies of the bonds in most of the covalent molecules (Pavia et al., 2010). IR absorption spectra were obtained on a Nicolet Is10 FTIR Spectrophotometer using KBr pellets in the range 400 to 4000 cm-1.
2.7. Photochemical stability and biological (bactericidal) activity of the complex [Cu (II) (PDTA)]-2 via carbonate and the mixture Na2[Cu (II) (PDTA)] + Na2SO4 via sodium salt
The photochemical stability of the APCA (PDTA) was measured by fluorescence spectroscopy in the UV-Vis region using a Cary Eclipse Spectrofluorimeter (Varian), while the bactericidal activity was evaluated according to the Kirby-Bauer method (Bauer et al., 1966; CLSI, 2006).
2.8 Direct sunlight photobleaching of the PDTA binder
1,3-Propylenediaminetetraacetic acid (PDTA) binder was converted to sodium form (Na4PDTA) by neutralization with NaOH, resulting in a pH ~ 13 solution. This solution was stored in a sealed clear glass container and subjected to 4 days of direct solar radiation. Fluorescence spectra were obtained at 25 °C at 24 h intervals. A Cary Eclipse Spectrofluorimeter (Varian) was utilized to determine the binder degradation. The sample was excited at a wavelength of 310 nm, and fluorescence monitored in the range of 320 to 700 nm.
2.9. Biological activity of metal complexes
Biological activity testing was performed by diffusion antibiograms disc according to the methodology proposed by Kirby-Bauer (CLSI, 2006). This assay measures the resistance of a bacterium to one or more antimicrobial agents for the analysis of the resistance to the biologically active compound. The biological activity tests with the [Cu (II) (PDTA)] complexes, obtained by sodium and carbonate synthetic routes, were carried out against the Gram-negative bacteria Erwinia chrysanthemi. This microorganism was isolated from cassava-salsa affected by soft rot and cultivated in the experimental agricultural area of the Faculty of Agronomical Sciences at the Federal University of Grande Dourados (Dourados, MS, Brazil).
The pre-culture started from a loopful of representative colonies present in primary isolation plate, which were inoculated in liquid MB1 broth at a rotatory shaker (0.85 rpm, 37°C). Bacterial growth was monitored by optical density using a spectrophotometer at 600 nm (OD600) up to 1.25. This OD was equivalent to 0.5 McFarland scale (1.5 108 CFU mL-1). Then, 100μL of the bacteria sus-pension was inoculated in Petri dishes containing the solid culture medium (MB1 agar). Subsequently, autoclaved paper discs of 6 mm diameter were introduced with the complexes at the concentrations of 10-1, 10-2 and 10-3 mol L-1 (M). These concentrations used are approximately equivalent to 36.3 g L-1 (3.63%), 3.63 g L-1 (0.363%) and 0.363 g L-1 (0.0363%). The paper discs impregnated with the complexes were clamped under the surface of the culture medium in the center of the Petri dish. The antibiotic Streptomycin 10 mcg / sensiobiodise / Cecon was utilized as control. All analyzes were carried out in quadruplicates for each concentration of the complex [Cu (II) (PDTA)]. After 48 h, the inhibition zones around the disks impregnated with the complexes and control were measured.
3. Results and discussion
3.1. UV-Vis molecular absorption spectroscopy
The results of the UV-Vis analysis of the metal complexes are shown in Figure 1. The displacements observed in the samples suggest the formation of the [Cu (II) (PDTA)] complexes according to the two synthesis routes: carbonate and sodium pathways. Differences in spectra shifts between H4PDTA, CuCO3 and [Cu (II) (PDTA)] ligand samples were evaluated.
The carbonate synthesized [Cu (II) (PDTA)]-2 complex showed intense absorption band in the spectrum between the 200 and 300 nm region (Figure 1A). The H4PDTA chelator in the free form had a band near 200 nm that was displaced after Cu (II) ion complexation (Figure 1B). CuCO3 showed two bands, in the ranges of 210 to 230 nm and 245 to 275 nm, respectively (Figure 1C), differing from the peaks obtained for the [Cu (II) (PDTA)] complex.
Similarly, the complex mixture Na2 [Cu (II) (PDTA)] + Na2SO4, synthesized via sodium showed an absorption band in the same region of the complex via carbonate, between 200 and 300 nm (Figure 1D). Thus, it is possible to suggest the formation of the Cu (II) complex in the two synthetic routes: sodium and carbonate. The observed band for the free-form PDTA ligand near 200 nm (Figure 1E) remained for the two synthetic routes. CuSO4∙5H2O presented two absorption bands, one near 200 nm and other at 250 nm (Figure 1F).
3.2. Infrared (IR) absorption spectroscopy
The infrared (IR) absorption spectra suggest the probable sites of complexation of 1,3-propylene-diaminetetraacetic acid (PDTA) to Cu (II) ion. The determination of the binder sites was performed by comparing the vibrational energies of the possible binder atoms, in this case the nitrogen and oxygen from the amino and the carboxylic groups of the binder. Table 1 presents the peaks experimentally obtained for the carbonate and sodium pathways.
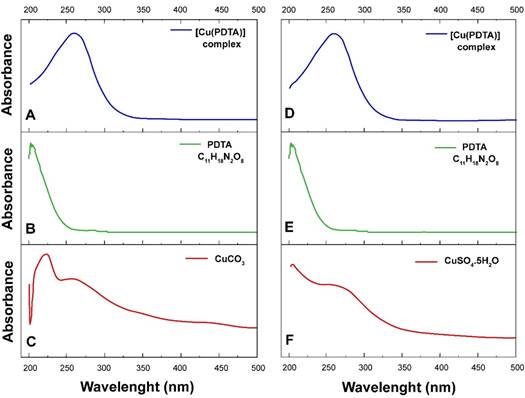
Figure 1 UV-Vis absorption spectrum for [Cu(II)(PDTA)]-2 synthesized via carbonate (A, B, C) and for the mixture Na2[Cu(II)(PDTA)] and Na2SO4 synthesized via sodium (D, E, F).
Table 1 Assignments of the FT-IR bands to the carbonate synthesized [Cu(II)(PDTA)] complex and the sodium synthesized Na2[Cu(II)(PDTA)] and Na2SO4 mixture
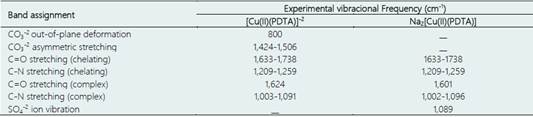
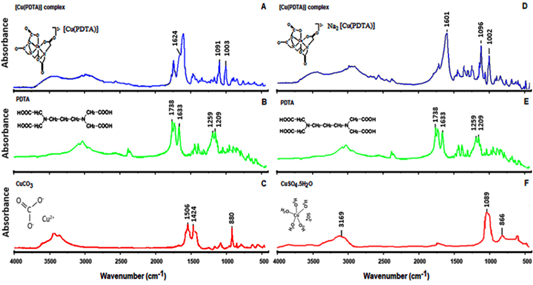
Figure 2 FT-IR spectra of the [Cu(II)(PDTA)] complex synthesized via carbonate (A, B, C) and of the mixture Na₂[Cu(II)(PDTA)] and Na₂SO₄ synthesized via sodium (D, E, F).
Figure 2A-C shows the IR absorption spectra for the carbonate synthesized complex [Cu(II)(PDTA)]-2. For carbonate synthesis, it is possible to observe in the spectrum, bands at 850-880 cm-1 characteristic of copper carbonate (CuCO3), due to the out-of-plane deformation υ2(A”2), and at 1,420-1,500 cm-1 due to stretching υ3(E”).
Figure 2D-F presents the IR absorption spectra for the Na2[Cu (II)(PDTA)] + Na2SO4 mixture via sodium. Starting from copper sulphate pentahydrate, CuSO4 5H2O, the band at 866 cm-1 refers to the vibrational mode of the coordinated water molecule, and at 3,169 cm-1 indicates the presence of uncoordinated water. The band at 1,089 cm-1 corresponds to the free SO4 -2 ion (Figure 2B). It observes the wave numbers (vibrational frequency) referring to the symmetrical and asymmetric stretches of the C=O and C˗N bonds, and the band displacement due to the complexation of the Cu (II) metal ion binding sites, which modifies the vibrational energies compared to the free (non-complexed) chelator. Spectroscopic characterization techniques suggest the formation of [Cu(II)(PDTA)] complexes obtained from 1,3-propylenediaminetetraacetic acid and Cu(II) (Figure 2).
These data are in strong agreement with the litera-ture, which reports CO3 -2 out-of-plane deformation at 850-880 cm-1, CO3 -2 asymmetric stretching at 1,420-1,500 cm-1, C=O stretching at 1,635-1,740 cm-1, C-N stretching at 1,200-1,250 cm-1, C=O stretching at 1,615 cm-1, C-N stretching at 1,000-1,100 cm-1, and SO4 -2 ion vibration at 1,050-1,100 cm-1 (Kagunya et al., 1998; Nakamoto, 2009).
3.3. Direct sunlight photodegradation
The PDTA ligand was excited at a wavelength of 310 nm, and the scan was performed in the range of 320 to 700 nm, presenting intense emission around 420 nm. After being subjected to successive periods of sun exposure, the PDTA ligand showed a decrease in fluorescence bands. The fluorescence spectrum of PDTA (Trilon F) as a function of expo-sure time to direct sunlight is shown in Figure 3.
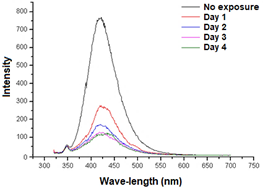
Figure 3 Fluorescence spectrum of PDTA (C₁₁H₁₈N₂O₈, Trilon F) for the study of the ligand's photochemical stability.
Experimental results showed that the reduction of the emission intensity of PDTA molecules is directly related to the degradation process by the formation of chemical intermediates and / or structures of different optical characteristics that contribute to the reduction of the emission intensity. The literature shows that the presence of carboxyl (-COOH), hydroxy (-OH), methoxy (-OR), amino (-NR2), cyanide (-CN) and sulfonic (-SO3H) substituent groups on the molecules may act as amplifiers of the fluorescence when present in fluorescent compounds (Lakowicz, 2006).
Experimental results suggest that photochemical degradation is related to molecular disruption and / or breakage of bonds involving the carboxylic and amino groups of the PDTA molecule with fluorescence reduction. The phenomenon at different intensities can be extended to the degra-dation of molecules of other APCAs. The study of photochemical stability was carried out only with PDTA ligand due to non-fluorescence of amino and carboxylic groups when coordinating the Cu (II) ion.
3.4. Biological activity of complexes [Cu (II) (PDTA)]
The antibiograms were performed for the two [Cu (II) (PDTA)] complexes, synthesized by sodium and carbonate synthetic routes, against Erwinia chrysanthemi. The antibiotic potential of Cu (II) complexes was evaluated by applications at concentrations of 10-3, 10-2 and 10-1M.
The results showed differentiated antibiotic activities, which have increased with the increase in the complex concentration from 10-3 to 10-1 mol L-1. However, bactericidal activity of the [Cu (II) (PDTA)] complex via carbonate showed higher bactericidal activity. The inhibitory effect on bacterial growth at 10-1 mol L-1 Cu (II) concentrations (PDTA) via carbonate showed an inhibition zone of 31 mm. This inhibition halo was larger than that observed with the antibiotic streptomycin halo (25 mm). Table 2 summarizes the inhibition halos for the carbonate and sodium complexes, in comparison with the positive (Streptomycin) and negative (acetonitrile solvent) controls.
3.5. Constant stability analysis of the [Cu (II) (PDTA)] and [Cu (II) NOM] complexes
In soil, natural organic matter (NOM) forms through the degradation of organic compounds, producing binders like humic, fulvic, citric, malic, fumaric, tartaric, and gluconic acids, which can compete for bioactive metals in plant nutrition, soil pathogen control, or microbiota activity (Feng et al., 2022). Using copper chelate [Cu(II)(PDTA)] over metal salts for pathogen control must account for toxicity, systemic transport, competition with NOM, and environmental impact reduction while ensuring metal availability to plants. Soil’s complex, heterogeneous nature complicates determining the stability constant for NOM complexes, such as humic and fulvic acids, as metal complex formation depends on the metal, binder, and competitors that may coordinate or precipitate the metal (Jaishankar et al., 2014). The stability of these complexes, expressed as log K, reflects the strength of coordination; higher log K values indicate stronger, more stable metal-ligand binding and increased likelihood of complex formation. Table 3 shows values calculated and / or estimated for the constant (K) for several complexes in homogeneous systems from soil organic complexants e.g. humic and fulvic acids, NOM and APCAs.
Metal chelates reduce metal reactivity, acting as carriers and releasers of micronutrients, ensuring their availability to plants under varying environmental conditions but dependent on soil pH. Chelates prevent metal precipitation in hard water, facilitate drip irrigation, and integrate with most pesticides due to their solubility and protective effects on metal species. Angelova et al. (1999) demonstrated that Cu(II) and Zn(II) mobility increases under moderately acidic conditions (pH 5-7) due to higher solubility and complexation with soil organic ligands, with micronutrient accumulation correlating directly to soil content. Brun et al. (2001) found that Cu(II) concentrations are higher in roots than aerial parts, with EDTA, DTPA, and ammonium acetate being the most effective extractants for evaluating bioavailability. EDTA showed a strong relationship with Cu(II) complexed by soil organic matter, extracting Cu(II) bound to organic sites, though its efficiency depends on soil management practices that influence organic complex stability and metal distribution (Nóvoa-Muñoz et al., 2007). However, EDTA is effective in calcareous soils but less suitable for acidic soils (Chaignon et al., 2003).
Table 2 Antibiotic and bactericidal activity of the [Cu(II)(PDTA)] complexes for Erwinia chrysanthemi (Gram-negative) control
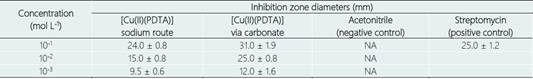
NA = no antibiotic activity.
Table 3 Stability constants (log K) for copper (II) complexes*
| Metal | Humic acid | Fulvic acid | MON | NTA | HEDTA | PDTA | EDTA | DTPA |
|---|---|---|---|---|---|---|---|---|
| Cu (II) | 8.65 | 4.00 | 6.90 | 10.60-12.68 | 17.40 | 13.00 | 18.80 | 18.80 |
*Estimated / calculated values for humic acid, fulvic acid and natural organic matter constants in the range of pH 5 to 6.5.
The availability of Cu(II) in soil depends on the amount of Cu(II) compounds added but decreases over time. This availability is influenced by humic acid content and changes in soil pH. Plant development is proportional to the increase in available Cu(II) and its interaction with fulvic acid. Copper toxicity is primarily related to free Cu(II) ions rather than total copper concentration. Toxic forms include Cu(II), CuOH⁺, and [Cu₂(OH)₂]²⁺, while copper bound to CO₃²⁻ is less toxic. Complexation with low molecular weight organic binders can increase toxicity, as these forms penetrate and accumulate in tissues more easily. However, when complexed with high-molar ligands like humic and fulvic acids or adsorbed onto particulates, copper toxicity decreases due to restricted passage through cell walls (Wang et al., 2009; Vašková et al., 2023).
Studies confirm that Cu and Zn preferentially form strong complexes with humic substances, particularly copper, as documented in the literature (Gimpel et al., 2003; Meylan et al., 2004; Rosa et al., 2007). While no stability constant for [Cu(II)(PDTA)] exists in the literature, it likely exceeds that of [Cu(II)(EDTA)] and NOM, following the APCA stability trend: NTA < EGTA < DCTA < EDTA < PDTA < DTPA < DOTA (Martins et al., 2005).
At an optimal pH range of 5.5-6.5 for parsley manioc, copper complexed with PDTA remains stable and available to plants, as the PDTA stability constant would surpass NOM-Cu(II). NOM may, however, act as a retention substrate, controlling Cu(II)-PDTA release and providing a gradual supply to plant roots. Meanwhile, humic substances form large, stable complexes with long soil residence times, reducing metal bioavailability, leaching, and efficiency in controlling soil pathogens or microorganisms.
4. Conclusions
Analyzes of UV-Vis and FTIR spectroscopic showed the formation of the metal complexes [Cu (II) (PDTA)]. This binder was rapidly degraded by sunlight and, thus, degradation products and / or metabolites can be rapidly recycled by the environment. An intense bactericidal activity was observed for the [Cu (II) (PDTA)] obtained by the two synthetic routes: carbonate and sodium pathways at 10-3 to 10-1 M concentrations. This activity may be related to the higher purity of the binder. The carbonate metal complex [Cu (II) (PDTA)]-2 had higher bactericidal activity in a greater zone of inhibition compared to the antibiotic Streptomycin. Thus, the [Cu (II) (PDTA)] and other [Cu (II) (APCAs)] complexes may be considered bactericidal and phytoactive compounds for the control of Erwinia chrysanthemi. The results may be enhanced in soils rich in decomposing organic material, where minimal competition or adsorption occurs with the organic matter, allowing the [Cu(II)(PDTA)] complex to remain stable, ensuring its availability and residual biological activity.















