INTRODUCTION
Melanoma is a tumor that originates from the transformation of melanocytes and melanoblasts after undergoing genetic mutations (Camargo et al., 2008; Moore et al., 2013; Phillips and Lembcke, 2013; Civita, 2017; INCA, 2018; Pimenta et al., 2023). In horses, melanoma represents 15% of skin tumors and affects all breeds, however, animals with dapple gray fur are more frequently affected due to genetic causes and factors such as the presence of dark skin and gradual loss of follicular pigmentation (Phillips and Lembcke, 2013; Teixeira et al., 2013; Civita, 2017). It is estimated that 80% of this group of animals over 15 years old will develop melanoma in one or several sites (Civita, 2017; Pimenta et al., 2023), including skin, oral cavity, uvea, perianal region, ventral surface of the tail, foreskin, corners of the lips, and eyelids (Moore et al., 2013; Phillips and Lembcke, 2013; Civita, 2017, Knottenbelt, 2016). It is reported that 90% of melanocyte tumors in horses are benign. Despite this, 66% of them progress to malignant forms with metastatic potential, which is a major concern for owners and veterinarians (Valentine, 1995; Smith et al., 2002,), Thus, diagnostic and prognostic tools are extremely important for the individual analysis of each case.
The diagnosis of neoplasia includes cytopathological and histopathological exams, which are the reference tests. However, when necessary, immunophenotyping and molecular techniques are used (Moore et al., 2013), which are important for the differential diagnosis of basal cell tumors, carcinomas, and melanocytic hyperplasia (Smith et al., 2002). These tumor cells can maintain melanin production causing an illusion of being less aggressive; nevertheless, this production can be reduced or absent, making the diagnosis difficult and, consequently, the therapy used, which can compromise the biological behavior of the lesion (Moore et al., 2013; Cavalleri et al., 2014).
To overcome conflicts in tumors with high degrees of dedifferentiation, immunophenotyping is used. This makes it possible to evaluate specific markers relevant to the diagnosis and prognosis; the tool helps in identifying abnormal cells, differentiating certain types, and establishing malignancy when histology alone is not enough (Ramos-Vara et al., 2008). Likewise, immunophenotyping allows for a concrete diagnosis, since the prognosis and treatment of the entity varies widely between benign and malignant forms (Knottenbelt et al., 2015).
The S-100 and Human Melanoma Black HMB-45 protein markers can be used to confirm the melanocyte lineage of the cells and investigate micro metastases in sentinel lymph nodes, which aids the diagnosis when it is hampered by the atypical presentation of cells in anaplastic or amelanotic melanomas (Cochran et al., 2006; Sternberg et al., 2010). In the case of S-100, a sensitivity of 97-100% and specificity of 75 to 100% have been reported (Kaufman et al., 1998; Trefzer et al., 2000; Dabbs, 2002; Pimenta et al., 2023); however, due to this lower figure for specificity, it is untenable for independent use for the diagnosis (Ohsie et al., 2008). In this way, it is necessary that the immunophenotyping result is always correlated with the histopathological analysis.
Other proposed biomarkers in equine melanoma include differentiation cluster 44 (CD44), E-cadherin, and Ki67. CD44 forms the transmembrane complex protein and is a fundamental component of the extracellular matrix (ECM) (Thorne et al., 2020). This biomarker is related to different physiological and pathological processes, and in tumors plays roles in the process of proliferation, adhesion, and migration (Ponta et al., 2003). Its deregulation contributes to the formation of different types of neoplasms (Xu et al., 2020), and some studies suggest that CD44 can be a promising clinical predictor (Ibrahim et al., 2019; Szczepanik et al., 2019). Thus, identifying the behavior of CD44s expression in equine melanomas can help in understanding the dynamics of tumor aggressiveness.
E-cadherin (ECAD) is a protein encoded by suppressor genes that participate in the cellular adhesion of melanocytes to keratinocytes, and some studies relate ECAD with prognosis in tumors (João et al., 2011). Its low expression may be related to the loss of cell adhesion, which has been associated with more aggressive tumor forms (Pannone et al., 2014). These facts are crucial to investigate its expression behavior in different types of tumors, including melanomas.
As for Ki67, it is used in melanomas to differentiate malignant from benign forms (Ramos-Vara et al., 2014) and in other tumors it has been used as an important prognostic marker, mainly because a high expression of Ki67 can indicate a poor prognosis for a patient due to higher rates of metastasis to lymph nodes (Nielsen et al., 2021). In this sense, its use in association with other biomarkers can serve as a subsidy to understand the biological behavior of different neoplasms.
In melanomas, knowledge of the mechanisms of carcinogenesis is crucial and the association of the expression of different molecules with histopathological findings is fundamental for better identification of possible biological behavior (Kaufmann et al., 1998). In equine melanomas, variations in Ki67, E-cadherin, and CD44 expression are related to the degree of aggressiveness (Seltenhammer et al., 2004; Moreira, 2013; Knottenbelt, 2016), however, there is still a need for more investigation about the prognostic value of these markers. Thus, the aim of this study was to verify cytological and histopathological parameters, as well as immunophenotyping with S-100, and prognostic markers Ki-67, E-cadherin, and CD44 to identify relationships between anatomo-clinical expression in equine malignant melanomas.
MATERIAL AND METHODS
Fifteen cases of melanoma in horses were included, confirmed by cytopathological and/or histopathological exams (biopsy), without predilection for breed, sex, or age, from the Archive of the Sector of Veterinary Pathology of the Faculty of Veterinary Medicine and Zootechnics of the Universidade Estadual Paulista «Júlio de Mesquita Filho» (FMVZ/UNESP), Protocol No. 0030/2018 - CEUA. Cytopathological samples were analyzed according to the presence of atypical melanocytes, mitosis, binucleation, and pseudo inclusion, among others, and classified according to frequency as scarce (25%), moderate (75%), or high (100%). The paraffin-embedded material was processed both for mounting histological slides stained with Harris hematoxylin and eosin (HE) and for the immunohistochemistry technique for S-100, Ki-67, CD44, and E-cadherin markers (Table 1).
In the analysis by optical microscopy (Carl ZEISS®), the «Breslow» micrometric criteria were applied with the following classification levels: superficial extensive melanoma (N1) for tumor cells contained only in the superficial papillary dermis; superficial extensive melanoma (N2) when it affects the superficial and intermediate papillary dermis; and infiltrative melanoma (N3) when it affects all layers of the dermis. The presence of lymphocytic infiltration, angiolymphatic invasion, necrosis, and mitosis was also considered.
For the immunophenotyping technique, equine skin, intestine, and mammary carcinomas were used as a positive control, serial sections of 4 µm for all samples and the following protocol: antigenic recovery with sodium citrate buffer solution (0.1 M; pH 6.0) in Pascal pressure cooker (Dako); endogenous peroxidase blockade with hydrogen peroxide (10% in PBS) for 10 min protected fromlight; proteinblockadewith skimmed milk (3% in 1x PBS) for 1 h; incubation of primary antibodies in a humid chamber at 4ºC (overnight); incubation with polymer (Easypath) for 20 min; development with DAB chromogen (3,32 -diaminobenzidine - Dako) protected from light for 5 min; counterstain with hematoxylin (Merck, Germany). Subsequently, the presence of cell receptors in the proliferative phase, loss of cell adhesion, and potential for migration and evasion were analyzed.
The density of immune stained cells was determined by counting in ten high-power fields (40x objective, 0.032 mm2) under an optical microscope (Carl ZEISS®) and expressed as a percentage. For the ECAD, CD44, and S100 expression the Intensity Reactivity Score (IRS) was used, where the staining intensity (SI) was assessed as negative (=0), weak (=1), moderate (=2), and strong (=3), and the reactivity was determined by the percentage of positive cells (PP) to KI67 tumors. Less than 10% positive cells were classified as having a low proliferation rate (PR), 11-50% positive cells as having a medium PR, and more than 50% positive cells as having a high PR.
In all cases, the analyses were independently performed by two pathologists and the differences were reassessed together. The images were obtained and saved usingAxionvision Rel 4.7 software, which was also used for the micrometric measurement of the samples. Finally, the results were submitted to descriptive and non-parametric statistical analysis in R program, with a significance level of p<0.05.
RESULTS
In total, 15 cases were reassessed by cytology and histology. Of the total, 4 (27%) animals were no defined breed (NDB), 3 (20%) Brazilian Saddle Horses, 4 (27%) Quarter Horses, and the others represented one each of different breeds.
The ages ranged from 4 to 24 years, with a greater number of cases being observed in animals up to 13 years old. There was no significant predominance regarding sex; 20% of cases were from necropsy and the highest number of records (26.7%) occurred in 2010. The sites affected by the tumor were the ocular conjunctiva, perianal region, and skeletal muscle (Table 2).
Table 2. Epidemiological data of horses with melanoma according to the Pathology Sector records (Universidade Estadual Paulista “Júlio de Mesquita Filho”, Brazil)
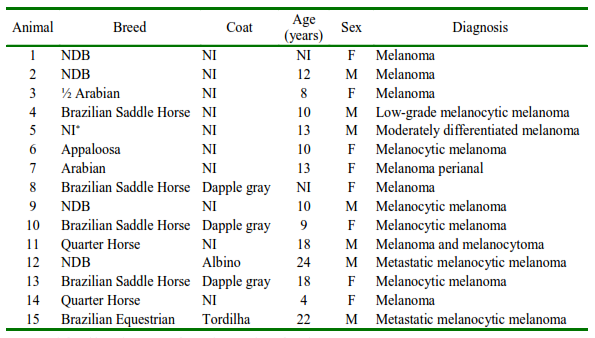
NDB: No defined breed; NI: not informed; M: male; F: female
Two animals presented metastasis, and in the first case, the lesions were diffuse in the cervical region, base of the tail, prepuce, eyelid, and left ocular region, while in the second case, the ocular conjunctiva, perianal region, and skeletal striated musculature were affected.
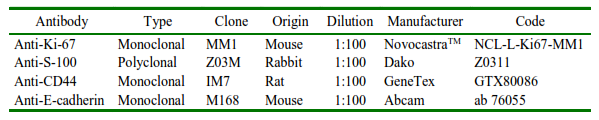
Based on the cytology criteria, all samples showed a high degree of malignancy criteria, cellular pleomorphism, some giant cells, increased nucleus-cytoplasm ratios, some sets of cells in the shape of a cluster, eccentric nuclei, blebs, anucleated cells, coarse chromatin pseudo inclusion (Figure 1), including cases of amelanocytic melanoma, which is highly aggressive.
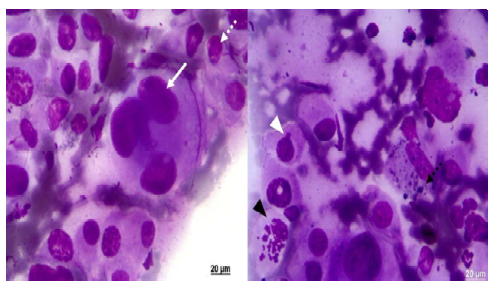
Figure 1. Malignancy criteria observed in equine melanoma cytology (ZEISS®). Pleomorphic cells with eccentric nuclei. Prominent nucleoli (dotted white arrow), multinucleated and giant cells (white arrow), proliferating cell (black arrowhead), bleb (white arrowhead), and granules in cytoplasm (dotted black arrow). Giemsa stain (FMVZ Record). Bar 20 µm
Considering the samples stained with HE (Figure 2), infiltration was observed in the skeletal striated muscle, cells close to the blood vessel, which is an indication of possible metastasis, as well as cells close to the adipose tissue. Pigmentation of cells by melanin was, in general, intense.
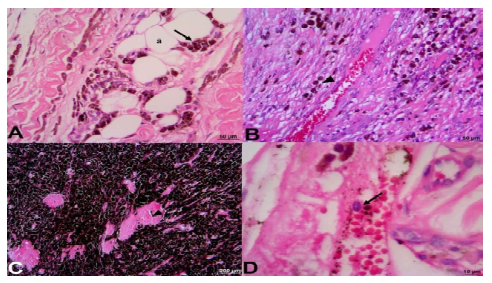
Figure 2. Equine melanoma (ZEISS®). A: tumor in skeletal striated muscle and tumor cells (arrow) close to adipose tissue (a); B: tumor infiltration in muscles and cells close to a blood vessel (arrowhead); C: infiltration in muscle tissue; muscle (arrowhead); D: cell inside a blood vessel (arrow). HE stain (FMVZ record). A and B, Bar 50 µm; C 200 µm,D 10µm
All tumors analyzed (Figure 3), even those without skin, were infiltrative (N3/TIII), since they were between 1.5 and 3 mm thick and the tumor cells reached all layers of the skin and the musculature, and there was an indication of metastasis in some cases due to vascular invasion. With respect to the immunophenotyping (Figure 4), CD44 was negative in all cases, the positivity rates for S-100, Ki67, and E-cadherin were 52.5%, 50.43%, and 10% respectively.
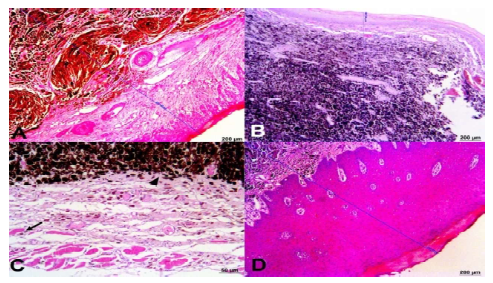
Figure 3. Equine melanoma. Infiltrative tumors in skeletal striated muscle. Infiltration measurement:A(2313.92 µm), B (1607.66 µm), and D (2247.65 µm); C: sample without the presence of skin, cells highly melanin-containing (arrowhead) and close to muscle tissue (arrow). HE staining (ZEISS®). Bar 200 µm; C 50 µm
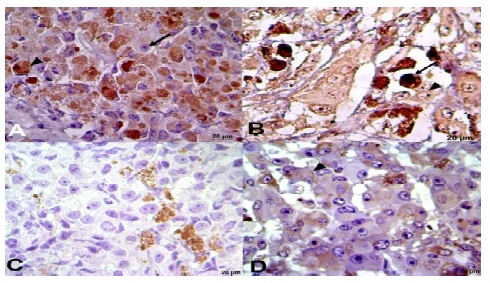
Figure 4. Immunophenotyping of melanoma in equines (ZEISS®) with Ki-67 markers. A: nuclear positivity - arrowhead and non-positive cell - arrow, S-100; B: nuclear and cytoplasmic positivity - arrow, CD-44; C: negative for membrane adhesion; brown staining from melanin), and E-cadherin; D: slightly positive for intercellular junction - arrowhead. (FMVZ Record). Bar 20 µm
DISCUSSION
The results of the cytopathological and histopathological examination showed that all the tumors were malignant and had different degrees of aggressiveness, including some infiltrating the skeletal muscles or being depigmented. These data support once again that both techniques are complementary for an accurate diagnosis and for establishing a suspected prognosis, but to supply the latter, the association with biomarkers is necessary.
In this way, when analyzing the different markers, it was found an absence of CD44 expression in all tissues. This result may be related to the metastatic potential or aggressiveness of the tumor, since the expression of this protein is proportional to the suppression of tumor progression and metastasis, according to Liu et al. (2014), likewise, because CD44 deregulation contributes to the formation of different types of neoplasms (Xu et al., 2020). Despite the limited number of samples, the information obtained is relevant, since in all tissues it was observed that the melanoma was infiltrative (N3/TIII) and had a thickness of 1.5 to 3 mm. These findings also indicate different degrees of malignancy, a criterion that in human medicine has been related to a poor prognosis (Thompson et al., 2012; GE et al., 2016; Taylor et al., 2018) and which could behave in the same way in horses.
Based on the data obtained, it is possible to assume that the absence of CD44 expression contributed to the development of mela-noma, especially in more aggressive forms, and even favored metastases, since one of the functions of CD44 is to control cell motility (Basakram, 2015), and the absence of expression would favor progression and metastases (Chen et al., 2018; Ma et al., 2022).
Despite these findings, the anti-CD44 antibody employed is generic and does not include some of the isoforms of this protein. In this way, and based on the hypothesis that the absence of CD44 expression contributes to the development of melanoma in horses, it is necessary to identify which CD44v isoforms are expressed in melanoma in this species, since their functions can be different and the potential to generate malignancy is variable (Prochazka et al., 2014; Ma et al., 2022), a fact already described in other tumors (Chen et al., 2018).
In other species, decreased E-cadherin expression has prognostic value and is associated with shorter survival (Kreizenbeck et al., 2008), however, in horses the role of ECAD expression is little known. This deserves special attention since some authors have demonstrated in other tumors that the loss of protein expression is related to malignancy (Asproni et al., 2015; Fonseca-Alves et al., 2015; Mestrinho et al., 2015; Kobayashi et al., 2018; Varallo et al., 2019). In the tissues analyzed, E-cadherin expression was low, which may be related to the metastatic and infiltrative potential of the tumor, as reported by Cavalleri et al. (2014). ECAD connects cells to each other via calcium-dependent homophilic binding (João et al., 2011); in this sense, it is important to remember that an important stage in the development of melanoma and metastasis includes the disruption of adhesions generated by E-cadherin between melanocytes and keratinocytes.
The decrease in ECAD expression and its association with malignant processes has already been described in oral dysplasia and squamous cell carcinomas (Huber et al., 2011; Chaw et al., 2012), in tongue tumors (Sakamoto et al., 2012), in breast cancer (Hazan et al., 2000), and in melanocytes. Ecadherin dysregulation has been demonstrated in studies comparing tissue from primary lesions and metastasis (Sanders et al., 1999; Sakamoto et al., 2012). In studies conducted in dogs, ECAD expression was higher in benign forms when compared to malignant forms (Silvestri et al., 2020). Likewise, Kreizenbeck et al. (2008) suggested that there may be an increase in survival time when there is an increase in cadherin expression, facts that deserve to be analyzed in new clinical studies in equine melanomas.
In all cases the proliferation rate was high, since the percentage of expression was always greater than 50%; in other tumors the Ki67 proliferation index has been shown to be useful in predicting behavior (Kop et al., 2020, Jacobsen et al., 2020, Dumitru et al., 2022). The high expression of Ki67 may indicate a poor prognosis in patients (Dumitru et al., 2022), and the same situation may occur in equine melanoma; however, it is necessary to investigate this expression in a comparative study with benign forms, which was not the objective of the current work. Thus, the immune expression of Ki67, together with the other biomarkers described, can help in establishing the prognosis, and it is necessary to investigate and associate them with histopathological parameters and, where possible, with metastasis to lymph nodes.
In the current work, taking into account the results in equine malignant melanomas, the absence of CD44 expression, and low Ecadherin and high Ki67 expression are more likely to be associated with malignant forms and possibly with lower survival rates. In this way, it is possible that the use of these markers in conjunction with less explored markers in the tumor, such as COX2 and PDL-1, may be an additional tool to evaluate the prognosis, especially in histomorphologically doubtful cases. However, further studies should be conducted to elucidate this hypothesis.
Finally, considering the S-100 protein, it is important in the identification of amelanocytic melanoma, is elevated in most patients with metastatic melanoma, and positivity in melanocytes must be both nuclear and cytoplasmic to be considered genuine (Kaufman et al., 1998). In the current work, the S-100 protein was expressed in 52% of the cases, which was in agreement with other works (Kaufman et al., 1998; Trefzer et al., 2000; Dabbs, 2002; Ohsie et al., 2008), however, due to the fact that some nonmelanocyte tumors and normal tissue express this protein, it was decided to correlate the histopathological findings and the marking to conclude the diagnosis.
In the epidemiological analyses of the cases, although few, some data demonstrated behavior like that described in the scientific literature.As with the coat, the most frequently described was the dapple gray, which is generally related to aging and genetic factors (Camargo et al., 2008; Sundstrom et al., 2012; Phillips and Lembcke, 2013; Civita, 2017). Likewise, the primary sites affected by the tumor are variable, and have already been reported at the base of the pinna, vulva, anus, base of the tail, lower eyelid, medial and lateral commissure of the eyeball, cervical region, perianal region, base from the ear to the jugular groove, and foreskin (Phillips and Lembecke, 2013; Moore et al., 2013; Knottbelt, 2016; Civita, 2017), which is in accordance with the finding of the current work, in which the primary site recorded in the clinical history was variable.
The age ranged from 4 to 24 years, with a greater number of cases being observed in animals up to 13 years old, while Knottbelt (2016) and Civita (2017) described that the affected animals are older (≥15 years old). In this way, the present results contribute to supporting the information that this is a disease of older animals, previously exposed for long periods of time to environmental factors.
Regarding the two animals with metastasis, unlike the present cases, the literature describes that metastasis is more common in the spleen, liver, kidneys, and lungs (Phillips and Lembecke, 2013; Civita, 2017). In this case, additional tests such as radiography, nuclear medicine, and magnetic resonance imaging are important, both for identifying metastasis and for choosing the therapeutic approach and prognosis.
In conclusion, the current work demonstrated that the results of cytopathological and histopathological examinations, including the application of the «Breslow» criteria and immunophenotyping, can help in identifying tumors with a higher degree of aggressiveness. However, due to the limited number of cases, it is suggested that it be investigated whether the same is repeated in a larger population. Despite this, it can be indicated that the use of CD44, ECAD, and Ki67 together with cytopathological and histopathological analyses should be performed to better establish the prognosis and therapy to be used, which, consequently, contributes to the prognosis of the animals. In addition, this study is important to complement morphological knowledge and immunophenotyping of melanocytic neoplasms in horses, that are currently little explored in the literature.












 uBio
uBio