Introduction
Biodiversity (bios in Latin = “life”; “diversitas” = “variety” (Wilson 1988)) is a widely used term with multiple dimensions. The concept was first introduced as “biological diversity” by Lovejoy (1980). The United Nations (2020) defines biodiversity as “the variability among living organisms from all sources including, interalia, terrestrial, marine, and other aquatic systems and the ecological complexes of which they are part; this includes diversity within species, between species and of ecosystems.” Biodiversity can refer to the number of documented species (~±2.3 million named extant species; Catalogue of Life 2023), or to the estimated number of species on Earth (which is highly variable; Stork 2018). It can refer to ecological diversity (e.g., honey-bee populations depending on plant diversity, Kaluza et al. 2018; parasitic aphids providing honeydew in exchange for protection by their ant hosts, Kudo et al. 2021), functional diversity (e.g., roles of dead trees and fallen logs in temperate forests, Franklin 1988), and genetic diversity (Gaston & Spicer 2004).
The fundamental challenge in estimating biodiversity is accurately assessing what species are present in an area (Magurran 2004). One key to improving species estimates is to survey cryptic (hidden) habitats where overlooked species or life stages may thrive (Rastorgueff et al. 2014, Sherrard et al. 2016). One type of cryptic habitats is small, accumulated pools of water-anthrotelmata, dendrotelmata, and phytotelmata. These attract research attention mostly when they pose epidemiological risks, particularly with Culicidae (mosquitoes). Anthrotelmata are temporary water pools unintentionally created by humans, such as discarded containers and old tires (Williams 2006, Oboňa et al. 2018). Dendrotelmata are pools collecting in tree holes formed by damage or rot to the trunk (Lozovei 1998, Campos 2013).
In this paper, we study communities living in phytotelmata, plant-containers that retain water and provide habitats for many organisms (Kitching 2000). It was believed that these types of hidden water-based communities were first noted by Chen Cangqi (=陈藏器) who was writing during the Tang Dynasty, China in the Ben Cao Shi Yi (=本草拾遗), observing a “mosquito-producing plant” (=wen mu cai=蚊母菜 (Chinese) (Frank & Lounibos 1983; Kitching 2000). However, we correct this misinterpretation that likely arose from a translation issue. In consultation with East Asian art history expert Kathleen M. Ryor, Carleton College, USA, we found several discrepancies. Copies of the Ben Cao Shi Yi itself are no longer extant; rather, the existence of the Ben Cao Shi Yi is known only due to translated fragments cited in Li Shizhen’s (=李时珍) Ben Cao Gang Mu (=本草纲目) from 1518-1593. In Luo Xiwen’s (2003) translation of Ben Cao Gang Mu, a bird by the name of “wen mu cao” (=蚊母草) is included (Li 2003: 3745), but no “mosquito-producing plant” is mentioned. There is a strong possibility that the name of this bird was mis-translated as a type of plant, because “cao” (草) means exactly “grass.” The context provided by this passage indicates that “mosquito-producing grass” is another name for this type of bird. Therefore, the earliest documentation of phytotelmata remains unknown.
Early historical studies revealed certain taxa associated with tree-holes (Dyar & Knab 1906), bamboo stumps (Dyar & Knab 1907), and bromeliads (Osburn 1913). Then Picado (1913) provided the first synthesis and comparison of various phytotelmata. However, Varga (1928) was the first to coin a specific term, ‘phytotelmata’ (Latin phyto = plant, telm = pool), to describe pitcher plant communities. For a history of research on these systems, see Frank and Lounibos (1983) and Kitching (2000).
Organisms commonly found in phytotelmata include odonates (Osburn 1913), many families of flies (Snow 1949, Belkin et. al 1971, Bradshaw 1983, Hayford et al. 2021), and beetles (Seifert & Seifert 1976, Frank & Lounibos 1983). Today, phytotelmata are regarded as ideal for studying ecology because they are small, temporary containers, have limited boundaries, and host relatively simple communities with fewer numbers of species. Some species spend their entire life cycle in these habitats and may have co-radiated with the plants, e.g., the rolled-leaf hispines beetles (Coleoptera: Chrysomelidae: Cephaloleiini; Wilf et al. 2000, Staines 2004, García-Robledo et al. 2013). These miniature ecosystems can be more easily manipulated for experiments, in comparison to larger ecosystems such as a tropical rainforest (Kitching 2000).
Different types of phytotelmata have been recognized (Fig. 1) (Picado 1913, Frank & Lounibos 1983, Kitching 2000). Bromeliad “tank” phytotelmata occur at the base of the leaves that form a rosette (Fig. 1A); the leaves even compartmentalize the accumulated pool of water. Various organisms (Greeney 2001), algae (Buosi et. al. 2014), insects, fungi, and frogs (Ruano-Fajardo et al. 2016) have been documented inhabiting bromeliad tanks. Insectivorous pitcher plants, Nepenthaceae and Sarracenieaceae, are another type of phytotelmata, where modified leaves hold a pool with digestive enzymes (Figs. 1B-1C; Bonhomme 2011, Ellison et al. 2012). Insects fall into the pitcher or are lured in and are digested to provide nutrients for the plants. However, some insects utilize the pitcher as a habitat (e.g., Diptera oviposit on the inner wall, Adlassnig et al. 2010). Bamboo (Poaceae) phytotelmata are formed when internodes are damaged, allowing water to accumulate (Figs. 1D-1E; Campos 2016). In many plants, leaf axils, where the leaf stem attaches to the main stalk, can retain water that provides a phytotelm habitat (Maguire 1971); Anosike et al. (2007) reported many mosquito species in axils of pineapple plants (Bromeliaceae) in Nigeria. Other phytotelmata may form in fallen fruit, fallen leaves, or seed pods (Fig. 1F; Kitching 2000). Zingiberales plants are renowned for hosting phytotelm communities in axils, leaf rolls, and bract pools (Figs. 1G-1I; Frank & Lounibos 1983, Kitching 2000).

Figure 1 Diversity of phytotelmata. A. Bromeliaceae, Trinidad and Tobago (photo: C.S. Chaboo). B. Pitcher plant (photo: D. Dendi). C. Pitcher (photo: D. Dendi). D. Bamboo, Puerto Rico (photo: D. Yee). E. Bamboo phytotelmata, Puerto Rico (photo: D. Yee). F. Fallen seed pod, Brazil nut (Lecythidaceae), Trinidad and Tobago (photo: C.S. Chaboo). G. Canna (Cannaceae), garden, Kansas City, U.S.A. (photo: D. Dendi). H. Musa (Musaceae), Peru (photo: H. Boyd). I. Heliconia (Heliconiaceae), Missouri Botanical Garden, U.S.A. (photo: D. Dendi).
We target here the phytotelm community of one species, Calathea capitata (Ruiz and Pav.) Lindl. (Fig. 2)(Zingerberales: Marantaceae). Zingiberales comprises over 2000 species in 92 genera and eight families (Kress et al. 2001, Christenhusz & Byng 2016). These are small to large herbaceous monocots; the order includes bananas and spices (e.g., cardamom, turmeric, and ginger) as well as cultivated ornamentals such as Heliconia and bird-of-paradise (Kress & Specht 2006). Zingiberales is the only plant order that potentially offers three types of phytotelmata: leaf rolls, leaf axils, and bracts (Staines 2011, Hayford et al. 2021). No extensive survey of leaf axil communities has been done (Ricarte et al. 2012). Wilf et al. (2000) dated the association of Cephaloleia histrionica beetles (Chrysomelidae: Cassidinae) in leaf rolls of Pitcairnia arcuata (André) André (Bromeliaceae: Pitcairnioidae) to the late Cretaceous period, ~100 million years ago. Researchers have experimentally altered various phyotelmata structures to study their impact on native fauna (Frank & Lounibos 1987, Naeem 1990); such experiments could potentially reveal larger scale changes in the ecosystem by modifying more feasible parameters.
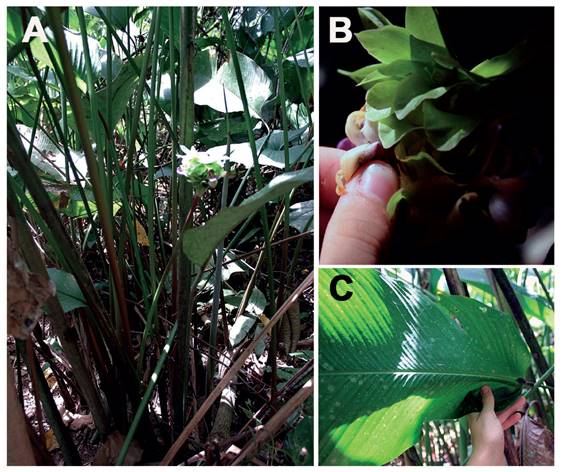
Figure 2 Calathea capitata (Marantaceae) in Peru (photos: T. Förster). A. Plant. B. Inflorescence. C. Leaf.
Our target study plant belongs to Marantaceae (31 genera, 530 species; Kennedy 2000, Ley & Bockhoff 2011). This lowland Neotropical family is commonly called “prayer plants” because of the unique movement of the leaves with the time of day (Herbert & Larson 1985). These plants are widely used in basketry, roofing, food wrapping, and as food (edible flowers, tubers, and arrowroot starch) (Hattori 2006). They support diverse pollinators of hummingbirds (Stiles 1978), butterflies (Kennedy 1978, Davis 1987), and bees (Ley & Bockhoff 2009). The genus Calathea G. Mey. comprises of 497 recognized species and is distinguished from other Marantaceae by the orientation of its leaf rolls (all pointing the same direction, in contrast to antitropic leaf rolls) (Eichler 1883, Schumann 1902, Kennedy 1978). Due to its attractive leaves and inflorescences Calathea is a common houseplant (Yang & Yeh 2008, Jalinsky et al. 2014, Rozali et al. 2014).
Many animals have been documented to use Calathea. Bees are frequent pollinators (Dodson 1966) and beetles feed on the leaves (Borrell 2005, Chaverri & Kunz 2006, Descampe et al. 2008, Etl et al. 2019). The bat, Thyroptera tricolor Spix (Chiroptera), has modified feet to use the rolled leaves of Calathea spp. as a roosting spot (Findley & Wilson 1974, Riskin & Fenton 2001). The hummingbird, Threnetes ruckeri Bourcier (Aves: Trochilidae), robs nectar from the inflorescences of Calathea lutea Schultes without accumulating pollen (Etl et al. 2019). There have been 23 studies targeting organisms in phytotelmata of 21+ species of Calathea (Table 1); these differ in taxon focus, collection method, and geography. Other taxa have been reported on Calathea, but it is impossible to determine the associated plant structure (see Staines 2004). Altogether, these studies reveal a diverse fauna living in bracts, such as flies (e.g., Ceratopogonidae, Fish & Soria 1978; Drosophilidae, Vaz et al. 2016; Periscelididae, Brandt 2019), protozoan ciliates that feed on algae (Wiackowski & Kocerba-Soroka 2016), and caterpillars (Lepidoptera) tended by ants (Horvitz & Schemske 1988). The leaf rolls are also inhabited by chrysomelid beetles (Strong 1977, García-Robledo 2013, Schmitt & Frank 2014, Jalinsky et al. 2014).
For the first time, we conducted an inventory, identification, and assessment of the phytotelmas community of C. capitata in the Peruvian Amazon to determine the presence of any taxa. Subsequently, we compared our results with those reported by Jalinsky et al. (2014) for C. lutea, which was studied in a nearby location using the same collection protocol.
Material and Methods
Our study is based on samples collected by author Timo Förster in Peru under Permit No. 0506-2011-AG-DGFFS-DGEFFS from the Peruvian Ministry of Agriculture and is funded by a U.S.A. National Science Foundation grant, EPSCoR #66928 to author CS Chaboo.
The study site is located at PERU: Province Paucartambo, Pilcopata, Villa Carmen Biological Station, 12°53'43.8"S, 71°24'13.7"W. This field station has recently been re-named the Manu Biological Station by its administration, the non-governmental organization (NGO), Amazon Conservation Association (amazonconservation.org). Caroline S. Chaboo trained Timo Förster on the protocols, and he conducted inventories for 9 months (October 2012 - June 2013) of the phytotelm communities of Zingiberales hosts at this site. The protocol and sample inventory codes of Caroline S. Chaboo survey are described elsewhere (Hayford et al. 2021).
The present study is based on a subset sample of the entire phytotelmata collections (many Zingiberales species); we study all the C. capitata samples and these comprise eight leaf rolls and eight bracts. We selected C. capitata so we could compare our findings with those of Jalinsky et al. (2014) which used the same collection protocols on a related species from a nearby site (162 km away, at a lower elevation, 225-296 asl). The biology of this host plant is presented under Results. Table 2 provides collecting information about each sample.
When leaf rolls contained a visibly high volume of liquid, the water was decanted into a zip-lock bag. The bag was placed into another zip-lock bag to prevent leakage and transported to the laboratory. For small volumes, all liquid was transferred in the field with a pipette into a screw-top measuring bottle with volume information. In the laboratory, collected leaf rolls were examined for additional liquids (if new had leaked) and, if necessary, a few ml was added to the field-collected volume or transferred to the measuring bottle if possible. The total liquid in each phytotelm was always thoroughly examined for specimens before disposal. The volume of liquid in leaf rolls was measured (n=7: 14, 28, 29, 35, 36, 49, 49 mL). The volume of liquid in the bract was too minute to be not measured with flask; the liquid accumulation in the bracts resembles a thin viscous film and we only noted if it was wet or moist.
The location of each sampled plant was recorded with a GPS model, Garmin eTrex 30. The accuracy of the recorded position is between 3 and 10 m (mostly 3m, only in very rough terrain and bad weather conditions it got up to 10 m). For altitude measurements, the internal barometric altimeter of the GPS device was taken directly at the position of the plant, with an accuracy of around 15 m.
Specimen Samples. The samples were collected by TF and the field information is provided in Table 2. These samples are deposited at Nebraska State Museum, University of Nebraska, Lincoln, U.S.A., and Universidad de San Marcos, Lima, Peru.
Literature Review. For historical context of phytotelmata and background information on C. capitata, multiple peer-reviewed journals from electronic research databases were used (Academic Search Premier, JSTOR, Tropicos, Google Scholar, and PubMed). Academic Search Premier is a subscription through the Johnson County Library, Leawood, KS. Subscription to JSTOR is through the Barstow School, Kansas City, KS. Literature search began in January 2021, and keywords or combinations of these keywords were used - biodiversity, anthrotelmata, dendrotelmata, phytotelmata, bromeliad, pitcher plant, tree hole, bamboo, leaf axil, Zingiberales, Marantaceae, Calathea, fauna, bract, rolled leaf. The literature review sought to find a Zingiberales species’ phytotelmata that was not yet evaluated and to establish past associations of insects with related plant species in the same genera, family, or order. Thus, our Table 1 assembles past studies that contribute original organismal associations with Calathea phytotelmata. Those studies exhibit different motivations, taxonomic focus, geography, and collections methods.
Specimen Processing. Specimens for pinning were first laid out to dry from their ethanol vials (for the beetles and the earwig). A steel pin was inserted through their mesothorax right of their line of symmetry. For smaller specimens, the Nikon SMZ800 was used to accurately insert the pin. Space was given under each pinned specimen for information on their collection site and identification. Identification slips for wet specimens (soft-bodied insects like Dermaptera, Diptera, Hymenoptera, Hemiptera; Araneae) are kept within their vials.
Specimen Imaging. Specimen imaging was done at the Enns Entomological Museum, University of Missouri, U.S.A., using the Leica MZ16 stereomicroscope with the Leica Application Suite v4.4 Extended Depth of Focus module. Pinned specimens were pinned on clay and surrounded by a vellum diffuser to omit unnecessary glare.
The microscope camera’s zoom and focus were adjusted to the correct levels, and the appropriate number of layered photographs were taken depending on each specimen’s depth. The layered photograph requires the user to identify the highest and lowest points of the specimen to focus on. Wet specimens were secured using hand sanitizer in a small dish, and only one layer of each was photographed. Images of plants and general collections were taken by the Olympus Tough TG-6. Images were edited and plated on Adobe Photoshop 2021 (version 22.4.3).
Specimen Identifications. All the different taxa were assigned a morphospecies and number (e.g., 1, 2, 3, etc.) so we could sort forms, identify as far as we could, and then obtain identifications later by taxon experts. These experts helped with refining some identifications: Mariana Chani Posse (Staphylinidae), Jon Gelhaus and Barbara Hayford (Diptera), Beulah Garner (Carabidae), Cristophe Girod (Dermaptera), Pedro Lozada (Cicadellidae), Joseph McHugh (Erotylidae), Diana Silva (spider), and Charles Staines (Chrysomelidae).
Results
Phytotelmata community of C. capitata. In our 16 samples (Table 2), we found 55 individuals of arthropods that were initially sorted to ~33 morphospecies (Table 3). All 16 sampled phytotelmata contained 1-21 individuals. These included Araneae (spiders) (Fig. 3A), Dermaptera (earwigs) (Fig. 3B), Hemiptera (bugs) (Fig. 3C), Hymenoptera (ants) (Fig. 3D), Coleoptera (beetles) (Figs. 4A-4F), and Diptera (flies) (Figs. 5A-5D). The most taxonomically diverse order was Coleoptera with six out of the 33 invertebrate morphospecies found (18.1%). Thirteen (23.6%) individuals were found in leaf rolls and 42 (76.3%) individuals were found in inflorescence bracts. Formicidae are the most abundant arthropod family in C. capitata in this study, making up 38.2% of individuals found. They could be said to have relatively moderate dominance within samples surveyed.
Table 3 Diversity of taxa found in leaf and bract phytotelmata of Calathea capitata (studied here) and Calathea lutea (Jalinsky et al. 2014). “?”=Diptera were not tabulated.

Figure 3 Arthropods found in phytotelmata of Calathea capitata (Marantaceae), Peru (photos: D. Dendi). A. Spider, Araneae: Corinnidae. B. Dermaptera: Spongiphoridae: Purex poss. frontalis (Dohrn, 1864). C. Hemiptera: Cicadellidae: Tenuicephalus sp. D. Hymenoptera: Formicidae.

Figure 4 Beetles (Coleoptera) found in phytotelmata of Calathea capitata (Marantaceae), Peru (photos: D. Dendi). A. Chrysomelidae: Cephaloleia sp 1. B. Cephaloleia sp 2. C. Cephaloleia sp. 3. D. Carabidae: Harpalinae: larva. E. Erotylidae: Xenoscelinae: prob. Loberus sp. F. Staphylinidae: Subfamily Tachyporinae, poss. Coproporus sp.
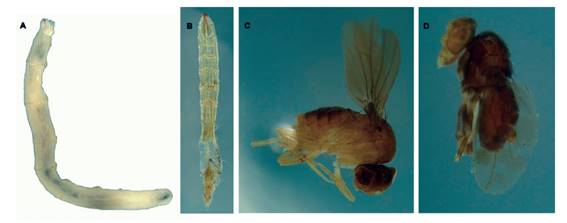
Figure 5 Diptera found in phytotelmata of Calathea capitata (Marantaceae), Peru (photos: D. Dendi). A. Limoniidae larva. B. Possibly Limoniidae larva. C. Drosophilidae. D. Undetermined.
Leaf-roll community of C. capitata. Thirteen individuals were found in leaf rolls, with seven (53.8%) from the chrysomelid beetle genus Cephaloleia Chevrolat, 1836. Dermaptera, Diptera, and Hemiptera were also present. Cephaloleia were more common in leaf rolls than bracts (7 out of 10 total individuals found). The ratio found of leaf-roll Diptera to bract Diptera is 1:3. Hemiptera and Dermaptera were found exclusively in leaf rolls. All individuals found in leaf rolls were adults. No Hymenoptera were found in our leaf rolls.
Bract community of C. capitata. The bract and leaf roll communities of C. capitata have a similar taxonomic diversity at the order level. The most abundant taxon in bracts are ants (Hymenoptera: Formicidae). Bracts also harbored exclusively the three spiders. Insect larvae were also only found in bracts, and in more abundance (16 individuals) than all orders except Hymenoptera.
Comparison of phytotelmata communities of two Calathea species, Peru (Table 3). Other historical studies (Table 1) on 21+ Calathea species did not target complete inventory of the phytotelmata community; many examined only Culicidae (mosquitoes) or just Chrysomelid beetles. There are other records of organisms using Calathea species as host plants (e.g., Staines 2004 lists the Cephaloleia beetle species) but these were random collections and not a systematic study of the phytotelmata - it is even unclear if these beetles were found in phytotelmata or were documented feeding on the open leaves.
We can directly compare our findings with that of Jalinsky et al. (2014) which was conducted at a different but nearby site (162 m between the two sites) in Peru’s Madre de Dios Department (12°34’08.0”S, 70°06’02.0”W) at a slightly lower elevation of 225 - 296 m. We note that Jalinsky et al. (2014) used the same protocols and surveyed 25 phytotelm samples total of their target plant, C. lutea, during a 2-week period, while we study 16 samples assembled over a 1-year period. Differences may be due to the different plant species, slightly different elevations (and correlated rainfall and temperature differences). Their study found 249 individuals (including 131 juveniles) in 18 morphospecies, whereas we found 55 individuals (including 19 juveniles) in 33 morphospecies. They found much greater taxonomic diversity on the family level. Like their study, we found arthropods in every sample. Beetles were dominant in their samples, but we found ants to be moderately dominant in our samples (38.2% of total individuals). They sampled many Diptera, but do not list all the families; interestingly, they found Culicidae only in leaf rolls and Syrphidae (rat-tail maggots) only in bracts (Radocy & Chaboo 2014). We found 21 ants, the most abundant individuals in C. capitata bracts. However, Jalinsky et. al. (2014) found only five individuals in C. lutea leaf rolls.
Discussion
Twenty-nine (52.7%) more individuals were found in C. capitata bracts than in leaf rolls. Diptera and Coleoptera were more common in leaf rolls than their bract counterparts.
Chrysomelidae beetles have been documented inhabiting leaf rolls in large numbers. For example, Schmitt and Frank (2014) found 301 individuals in 120 leaf rolls from 18 Zingiberales species (three are Calathea spp.) in Costa Rica. Jalinsky et al. (2014) found 227 individuals in 19 leaf rolls of C. lutea; they found no chrysomelids in the bracts of C. lutea and just two adults in eight bracts of C. capitata. Chaboo and Staines (2015) indicate 38 species of Cephaloleia for Peru; all are likely using Zingiberales hosts but their biologies are largely unknown (Staines 2004). Aristázabal et al. (2013) noted the concern of local Colombian farmers about coleopteran pests of their Zingiberales crops, grown to export flowers.
We found four Dipteran adults, all in leaf rolls of C. capitata. Jalinsky et al. (2014) found many Diptera in both bracts and leaf rolls of C. lutea. Flies and their larvae are commonly classified as detritivores (e.g., the eating patterns of phytotelmata inhabiting Psychodid larvae; Bravo et al. 2014).
Dermaptera (3 individuals) were exclusively found in leaf rolls in both studies. This could imply that Dermaptera inhabit Calathea leaf rolls for either habitat or feeding, but more studies on this matter are required for any conclusion. Earwigs can be omnivorous, feeding on arthropods and inflorescences alike, or carnivorous (Orpet et al. 2019).
One cicadellid bug as well as one nymph were found in C. capitata, while none were found in C. lutea. Cicadellidae are strictly herbivorous, feeding on plant sap with their piercing and sucking mouthparts (Leopold et al. 2003). The C. capitata leaves could be a more adequate source of plant sap than those of C. lutea. The nymph was found in the bract, whereas the adult was found in a leaf roll, implying different life stages use differing habitats on the same plant.
Hayford et. al. (2020) evaluated the Dipteran community for a few C. capitata samples; however, our study is the first to investigate the broader arthropod phytotelmata community of this plant species. These findings could be useful for ecological surveys and experiments in the future.
The 16 larvae of Coleoptera, Diptera, and Hemiptera were found exclusively in bracts. The taxonomic membership of the communities we found may differ seasonally and by habitat over the geographic range of the host plant. Information on the host plant C. capitata itself was scarce.
Our study supports Jalinsky et al. (2014)’s hypothesis that adult and larval insects, such as chrysomelids, exhibit more flattened bodies due to living in a phytotelma habitat. Jalinsky et al. (2014) also suggested that “thick gelatinous fluid” they found in the C. lutea bract could explain the relative paucity of fauna, however, we found more individuals in bracts. This could mean that either C. capitata possesses less of this fluid or the fluid provides a source of nutrition.
Gaps in data. While leaf axils were found to be a viable phytotelm type in Zingiberales (Hayford et al. 2020), C. capitata leaf axils were not sampled here. Populations of bacteria, algae, viruses, fungi, and endoparasites were also not examined in our dataset but could impact ecosystem structure and roles. The sample size, both geographically and numerically, was relatively smaller than that of Jalinsky et al. (2014). As only samples from one site were taken, C. capitata must be surveyed in other areas of Peru and South America and in different seasons for more conclusive data.
The ecological roles of the different taxa in phytotelm communities are largely understudied. We know the chrysomelids are herbivores whereas the other species are mainly predators or detritivores (e.g., Diptera larvae). Some may be short-term visitors and others, like Chrysomelidae, are residents.
More than 100 years ago, Picado (1913) noted the long list of taxonomic experts that are needed to determine detailed identifications of species in phytotelmata. This issue of the “taxonomic impediment” (Rouhan & Gaudeul 2021) is still relevant today, constraining levels of taxon identifications. Our study demonstrates that C. capitata enriches local diversity (i.e., the rainforest) by providing a habitat, food source, and nursery for diverse arthropods. The next step in assessing phytotelm biodiversity would be to survey more plants of C. capitata and determine any seasonal variations in its diversity (e.g., changing population structure, taxonomic membership, or turnover). Further study can reveal how phytotelmata interact with the entire forest (e.g., how they contribute to the total diversity). The phytotelmata structure itself has a discrete life cycle: a beginning, middle, and end; the inflorescence senesces, and the leaf roll opens completely, no longer holding water. It is highly possible that phytotelmata-inhabiting organisms are evolutionarily constrained by this dynamic habitat.












 uBio
uBio