Introduction
The expansion of the agricultural frontier has resulted in the depletion of high-quality forests, leading to biodiversity loss and alterations in fauna composition (Carrillo et al. 2007, Murillo et al. 2016). One of the most threatened ecosystems in Ecuador is the dry forest, which occurs in continuous stretches along the coast and in isolated valleys within the Andean region (Troya 2016). This ecosystem has been subjected to human pressure, including non-sustainable agricultural practices such as weed burning, the intensive use of pesticides, and intense livestock farming (Hernandez Camacho 1992; Laurance & Bierregaard 1997, Amador & Martínez 2011). These practices have put the ecosystem at risk and threaten the survival of the species that depend on it. The Manabí province forest, which belongs to the Cordillera Chongón Colonche ecosystem, has experienced secondary succession for at least 40 years, resulting in the re-establishment of the native dry forest vegetation in abandoned croplands (Pourrt 1998). Allowing for secondary succession is of great importance to promote a healthier ecosystem through natural processes, leading to more diverse composition of flora and fauna compared to productive lands (Caamal-Maldonado & Armendariz-Yañez 2002). A case in point is Cascol town in Manabí province, situated at the center of the Ecuadorian coastal region, where landscape regeneration has been ongoing over the last four decades.
While existing biological research has primarily focused on botanical and ecological aspects of these ecosystems, there has been relatively limited investigation into insect populations (Troya 2016, Moreira & Arias 2019). Insects plays an important role in ecosystem health, meaning that changes to their population and species composition can have significant impacts. Coleopterans, a particularly abundant group of insects, serve as excellent models for studying ecosystem health. The use of coleopterans as bioindicators of ecological disturbance and forest health is based on the ability of some species to respond rapidly to environmental changes caused by anthropogenic activities (Noss 1990, Halffter 1993, Celi & Dávalos 2001, Albrecht 2003, Pearce & Venier 2006). These organisms are extremely sensitive to environmental alterations such as pollution, plant succession, and ecosystem degradation (Escobar & Chacón 2000). Landscape degradation, such as monocultures, may affect their physiological performance and ecological preferences, triggering species displacement towards healthier ecosystems, sensitive species disappearance, or an accelerated increase of highly adaptable species (Noss 1990, Wink et al. 2005). Conversely, landscape regeneration tends to foster increased insect diversity or recovery of specific groups, compared to insect communities in agricultural settings and plantations. For example, both Miss and Cuauhtemoc (2007) and Alvarado and Osorio (2020) noted an increase in the diversity of coprophagous and saproxylophagous beetles in regenerating primary and secondary forests, coinciding with vertebrate mobility and natural vegetation decomposition.
The study of beetle assemblages in farmland and secondary forests is essential for assessing ecosystem health and the dynamics of beetle communities (Muñoz et al. 2015). Furthermore, for any conservation initiative to be effective, it is necessary to have basic information of the diversity of beetles and the experimental designs applied for monitoring (Caamal-Maldonado & Armendariz-Yañez 2002). This background motivated the present work, focused on the fragmented landscape of the Cascol (Manabí province); we present preliminary information on the diversity of tropical dry forest beetles by comparing cropland and secondary forest in regeneration. This research aims to contribute to a better understanding of the beetle communities in this ecosystem to reinforce conservation efforts in the region.
Material and methods
Study sites and sampling. Field work took place in December 2020, in El Descanso community (1°44´S, 80° 29´ W, 555 m of altitude) located in Cascol, Paján Municipality (Manabí province), within the Cordillera Chongón Colonche (CCHC) ecosystem in the center of the Ecuadorian coastal region (Bonifaz & Cornejo 2004). The sampling period overlapped with the beginning of the rainy season. Cascol vegetation cover is characterized by tropical dry forests; the annual rainfall in this area is 2500 mm and has an average annual temperature of 24 °C (Muñoz et al. 2015).
Nine transects of 200 m long were established in three sampling sites (three transects per site) covering an approximately 30 ha total area: two inorganic corn croplands and one secondary forest (Fig. 1). Agrochemicals are used for pest control and fertilization in the cropland; however, during the entire survey no chemical compounds were applied. The forested area has been through a regeneration process for about four decades. Transects were separated between each other by 20 m and were all placed in the center of the property to avoid edge effects. 20 pitfall traps were placed per transect, every 10 m, and were checked every day. Traps remained active for one month. Three types of bait were used to attract beetles based on their feeding preferences: The first week no bait was used as a control setting, the second week decomposing fish was used to attract necrophagous beetles, the third week decaying fruit was used for saprophagous beetles, and cow excrement was used for coprophagous beetles the fourth week. We also performed active search (manual collection) for beetles hidden under stones, in leaves, flowers, tree branches, shrubs and decaying dry trunks for complementary diversity inventory. Specimens were collected in Ziplock bags with 75% alcohol, labeled and transferred for processing and identification. In the laboratory, a Motic SMZ-171 stereomicroscope, taxonomic keys, and the reference collection of the QCAZI museum were used for specimen identification to the most specific taxonomic level possible within the scope of the study (Erwin 1991, Borror et al. 1992, Morón 1997, Martínez 2005, Reyes & Morón 2005). All specimens collected were identified to family and catalogued as morphospecies, and when possible, specimens were identified to genus. The specimens were collected under the Ministry of the Environment, Water and Ecological Transition permit MAAE-ARSFC-2020-0879 and reference specimens were deposited in the dry collection of the Museum of Invertebrates at Pontificia Universidad Católica del Ecuador (QCAZI) (Appendix 1).
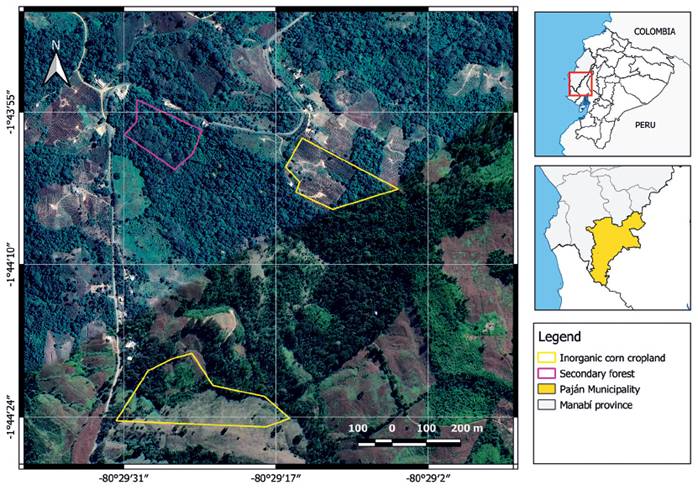
Figure 1 Study area in El Descanso, Manabí, Ecuador. The croplands and secondary forests where the beetle assemblages were evaluated are shown.
Sampling effort estimation. To establish the representativeness of the sampling, morphospecies accumulation curves and sample coverage analysis was used at each site and for each type of bait, using the number of pitfall traps as sampling unit, with the non-parametric estimator CHAO 1 (Colwell et al. 2012, Chao et al. 2014, Chao et al. 2016). Additionally, a sampling completeness analysis (sample coverage) was performed, which calculates the proportion of the individuals’ richness, by using the iNEXTLA program (Chao & Jost 2012).
Alpha diversity. We estimated the relative abundance of morphospecies per site. The Simpson index 1-D (λ) was used to calculate the probability that randomly collected individuals are of the same morphospecies. This index ranges between 0 and 1, where values close to 0 represent greater dominance and less diversity, and values close to 1 represent less dominance and greater diversity. Also, Shannon index (H') was used to calculate the diversity per site. The value obtained ranges between 0 and 5; where 1 is considered a low diversity site and above 3 corresponds to high diversity (Magurran 1988).
Beta Diversity. Taking into consideration morphospecies variation between sites, a multivariate cluster analysis was performed to estimate beta diversity using the distance coefficients of Bray-Curtis, contemplating the number of individuals per species collected (Moreno 2001). A two-way permutational multivariate analysis of variance (PERMANOVA) was used to test community structure differences within sites (secondary forest and cropland) and between bait categories, based on Bray-Curtis’s dissimilarity distance. A multidimensional scaling analysis was utilized to visualize the dissimilarity described. Both diversity index and comparative analysis were performed using Past V. 0.4 software (Hammer 2001).
Results
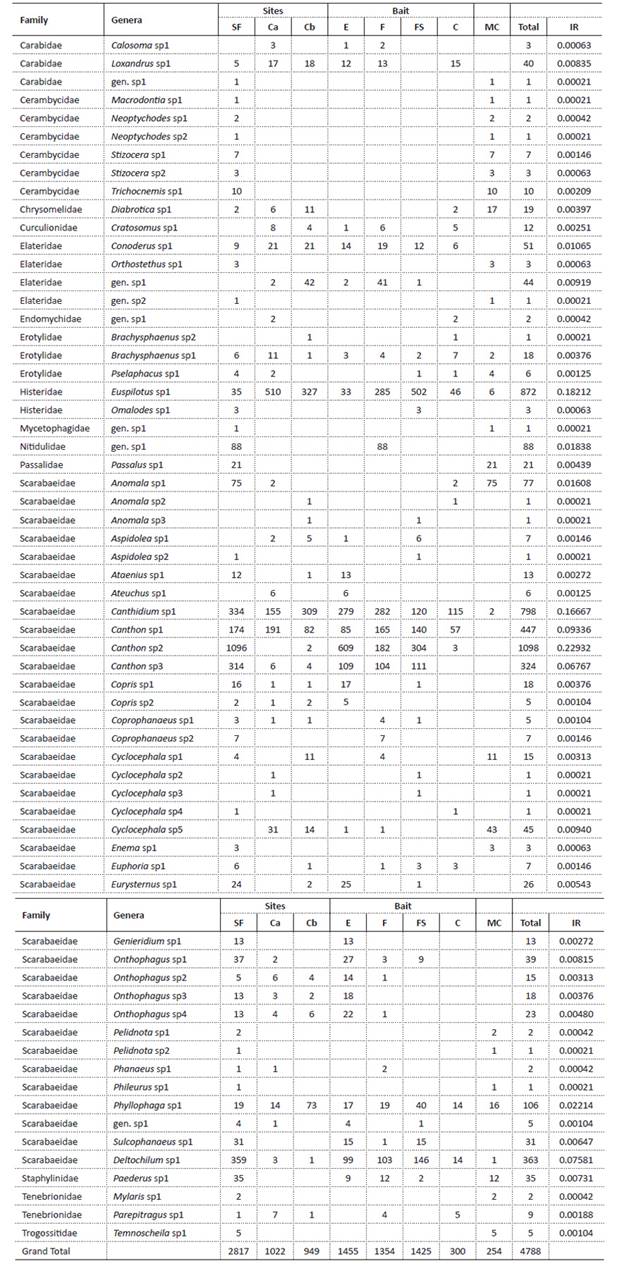
Morphospecies richness and diversity. We collected 4788 beetles of 64 morphospecies classified within 15 families. 254 individuals came from manual collections belonging to 28 morphospecies, 21 of which were also found in the pitfall traps (Table 1).
Table 1 Abundance of coleopteran species collected in secondary forest (SF) and two cropland sites (Ca and Cb). We also report the abundance of coleopterans according to the bait type where the taxa were observed: Excrement (E), Decomposing fruit (F), Decomposing fish (FS), Control (C), and manual collections (MC) and the total relative importance (RI) of each group.
The morphospecies accumulation curve almost reached the estimate asymptotic phase in the secondary forest for all bait types. According to the CHAO 1 criteria, 100% of collection efficiency was observed using fish and fruit baits, 87.5% with excrement and 91.1 with no bait in the secondary forest, where the maximum number of species expected was 24 (Fig. 2). In agroecosystem, the maximum number of species expected was 15; according to the CHAO 1 criteria, 100% of collection efficiency was observed with no baits while lower efficiency was observed will all bait types: 72.9% and 77.1% with fish, 89.7% and 72.3% with fruit, and 91.7% and 96.4% with excrement Fig. 2). The sample coverage analysis showed a high percentage in secondary forest (Excrement = 99%, fruit = 98%, fish = 99%, no bait = 1%) and in both croplands (Excrement = 95/97%, fruit = 97/96%, fish = 95/97%, no bait = 0.98/1%).
Overall, there was low dominance and high diversity in all sites (1-D sf = 0.799; 1-D a = 0.691; 1-D b = 0.758). The forest had both the greatest diversity and abundance (H' sf = 2.207, spp sf = 52, N sf = 2817) compared to cropland (Ha' = 1.695, spp a = 31, Na = 1022; Hb' = 1.857, spp b = 29, Nb = 949).
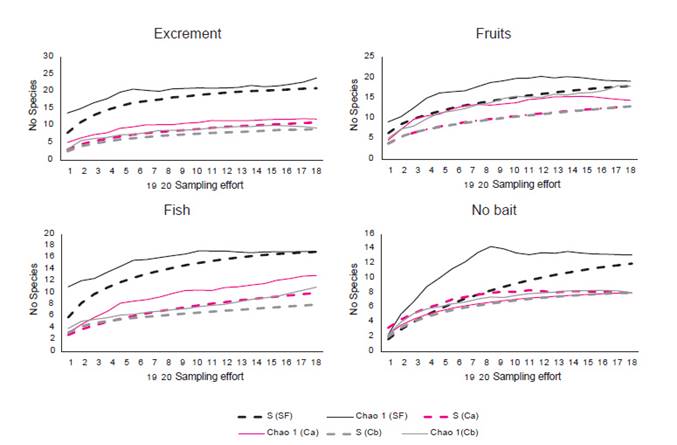
Figure 2 Species accumulation curve considering the number of pitfall traps as sampling effort in Secondary Forest and both Cropland sites (a and b) while sampling with no bait and with three bait types (excrement, fruit, fish). Dashed indicates the actual values, and continues line indicates the values of Chao 1.
The most representative family in the secondary forest was Scarabaeidae including Canthidium (Erichson 1847) sp1, Canthon (Hoffmannsegg 1817) sp1, Canthon sp2, Canthon sp3 and Deltochilum (Eschscholtz 1822) sp1. On the other hand, in the cropland the most abundant groups were Histeridae, Euspilotus (Lewis 1907) sp1 and Scarabaeidae (Canthidium sp1, Canthon sp1) (Table 1). Some morphospecies were exclusively found in one type of habitat; this was the case of 28 morphospecies which occurred only in secondary forest such as Carabidae gen. sp1, Macrodontia (Lacordaire 1830) sp1, Neoptychodes (Dillon & Dillon 1941) sp1 and sp2, Stizocera (Audinet-Serville 1834) sp1 and sp2, Trichocnemis (LeConte 1851) sp1, Orthostethus (Lacordaire 1857) sp1, Elateridae gen. sp2, Omalodes (Erichson,1834) sp1, Mycetophagidae gen. sp1, Nitidulidae gen. sp1, Passalus (Fabricius 1792) sp1, Aspidolea (Bates 1888) sp2, Ataenius (Harold 1867) sp1, Cyclocephala (Dejean 1821) sp4, Coprophanaeus (Olsoufieff 1924) sp2, Enema (Hope 1837) sp1 and sp2, Genieridium (Vaz-de-Mello 2008) sp1, Onthophagus (Latreille 1802) sp1, Pelidnota (MacLeay 1819) sp1 and sp2, Phileurus (Latreille 1807) sp1, Sulcophanaeus (Olsoufieff 1924) sp1, Paederus (Fabricius 1775) sp1, Mylaris (Pallas 1781) sp1, Temnoscheila (Westwood 1830) sp1; while 12 morphospecies such as Calosoma (Weber 1801) sp1, Cratosomus (Schoenherr 1825) sp1, Elateridae gen. sp1, Endomychidae gen. sp1, Brachysphaenus (Lacordaire 1842) sp2, Anomala (Samouelle 1819) sp2 and sp3, Aspidolea sp1, Ateuchus (Weber 1801) sp1, Cyclocephala sp2, sp3, and sp5 were only observed in crops (Table 1).
Bait association. Most of the morphospecies were observed in multiple bait traps; however, several were exclusive to one type of bait. Ataenius sp1, Ateuchus sp1, Copris (Geoffroy 1762) sp2, Onthophagus sp3 and Genieridium sp1 were observed only in excrement traps. Nitidulidae gen. sp1, Coprophanaeus sp2, Cyclocephala sp1, and Phanaeus (MacLeay 1819) sp1 were found in fruit exclusively. Omalodes sp1, Anomala sp3, Aspidolea sp2, Cyclocephala sp2 and sp3 were exclusive collected in fish bait traps.
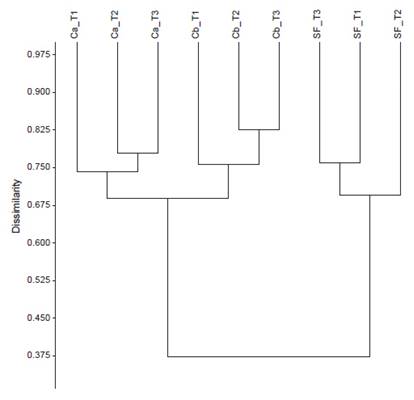
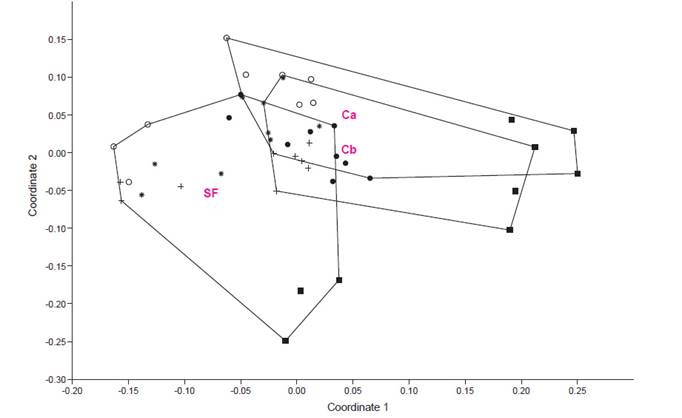
Community structure. The analysis of dissimilarity shows aggregation of morphospecies based on the level of disturbance (Table 2, Fig. 3); the coleopteran composition of the secondary forest differs from the coleopteran composition of both cropland sites which are grouped within a unique cluster.
Table 2 Dissimilarity matrix based on Bray Curtis Index (Figure 3).
| SF_T1 | SF_T2 | SF_T3 | Ca _T1 | Ca _T2 | Ca _T3 | Cb _T1 | Cb _T2 | Cb _T3 | |
| SF_T1 | 1 | 0.668 | 0.759 | 0.33 | 0.45 | 0.44 | 0.51 | 0.46 | 0.47 |
| SF_T2 | 0.668 | 1 | 0.723 | 0.25 | 0.35 | 0.3 | 0.41 | 0.32 | 0.34 |
| SF_T3 | 0.759 | 0.723 | 1 | 0.26 | 0.37 | 0.36 | 0.37 | 0.34 | 0.36 |
| Ca_T1 | 0.334 | 0.251 | 0.257 | 1 | 0.74 | 0.74 | 0.57 | 0.66 | 0.6 |
| Ca _T2 | 0.449 | 0.348 | 0.37 | 0.74 | 1 | 0.78 | 0.68 | 0.71 | 0.72 |
| Ca_T3 | 0.443 | 0.305 | 0.362 | 0.74 | 0.78 | 1 | 0.72 | 0.82 | 0.73 |
| Cb_T1 | 0.511 | 0.413 | 0.372 | 0.57 | 0.68 | 0.72 | 1 | 0.72 | 0.79 |
| Cb _T2 | 0.458 | 0.324 | 0.343 | 0.66 | 0.71 | 0.82 | 0.72 | 1 | 0.83 |
| Cb _T3 | 0.467 | 0.339 | 0.36 | 0.6 | 0.72 | 0.73 | 0.79 | 0.83 | 1 |
The abundance of coleopterans was significantly different between sampling sites and baits (F (2,8) = 12.43; p < 0.001) with a higher abundance in the secondary forest than from both cropland sites (Fig. 2). Manual capture abundance also differed from the one using baits (F(2) = 12.39; p < 0.001). Several groups were found to feed on multiple bait resources, which shows similar composition (Fig. 4); excrement was the bait yielding the highest abundance in the forest while fruit and fish yielded the highest abundance in cropland.
Discussion
In ecosystems exposed to alterations, whether natural or human-caused, species diversity tends to reduce as sensitive organisms disappear, and tolerant organisms abundance increases (Metcalfe 1989). However, the regenerating forests situated between altered areas, like croplands, are expected to be refuges for entomofauna and harbor higher diversity concentrations (Bihn et al. 2008, Moulatlet et al. 2021). Our study presents the first preliminary assessment of the beetle diversity in tropical dry forests of Manabí, where one of the main problems is the continuous fragmentation of vegetation cover.
We found 64 beetle morphospecies, 52 of which were found in secondary forests and 31 in croplands. Canthon sp2, sp3, and Deltochilum were dominant in secondary forests, while Euspilotus sp1 and Canthidium sp1 were dominant in croplands. Euspilotus and Canthidium are known to tolerate disturbance and have been associated with fragmented ecosystems (Escobar & Chacon 2000).
We report a representative number of beetles morphospecies in comparison with other disturbed dry forest ecosystems in the Ecuadorian coast and Andean region. For instance, Dominguez Marin-Armijos and Ruiz (2015) found six species in dry mountain shrub in Catamayo-Loja, Molina-Moreira and Arias (2019) recorded nine species, while Ramos and Sanchez (2019) discovered seven species in secondary forests of Calceta-Manabí that are characterized by dry forest vegetation; this locality is geographically closer to Cascol and has a similar topography.
In addition, Cascol has a higher diversity of beetles than similar ecosystems in Colombia and Peru. Uribe and Vallejo (2013) reported 22 species in a tropical dry forest of the Magdalena River. Andrade et al. (2017) found 31 species in farmland, disturbed riparian vegetation and native dry forest habitat in Peru.
Although the species accumulation curve and the completeness analysis suggest an efficient monitoring performed in both ecosystems, the estimators of CHAO 1 suggest that there are still morphospecies to be recorded, in both forest and crop. Complementary capture techniques, such as flight interception traps, light traps, and canopy fumigation, as well as a more extensive monitoring throughout the year should be implemented to provide a full beetle species inventory and a comprehensive analysis of the beetle community (Pedraza et al. 2010, Otavo et al. 2013).
The dissimilarity dendrogram shows differences in beetle composition between croplands and secondary forests, with greater morphospecies diversity and richness observed in the regenerated forest site. Scarabaeidae morfospecies such as Canthidium sp1, Canthon sp2, and Deltochilum sp1, mainly associated with excreta and decaying fish, were more abundant in the secondary forest, although not exclusive to this habitat. In general, this family is associated with better quality forest due to its significant role in organic (animal-origin) matter decomposition, indicating better quality of regenerated landscapes (Chamorro et al. 2019). Conversely, fewer morphospecies were observed in croplands, and only two morphospecies, Euspilotus sp1 and Canthidium sp1, were dominant. These genera are considered ecologically tolerant to fragmentation (Reyes Novelo et al. 2007).
We detected certain morphospecies previously reported as pests, such as Phyllophaga (Harris 1826) species that tend to destroy plant roots (Cueva 2014, Contreras 2019, Morocho et al. 2020). Similarly, Diabrotica (Chevrolat 1836) species feed on foliage, flowers, and roots causing physiological alterations and delaying crop development (Pérez et al. 2006). Diabrotica contains the largest number of pest species, targeting some of the most important horticultural crops on the continent (Cabrera Walsh 2005, Cabrera Walsh et al. 2020). In fact, corn and other grasses could be the most important larval hosts, which has helped this group become widespread. Although these species were expected to be found only in crops, we also found them in the secondary forest. Pests that are widely distributed are recognized for their ability to feed on multiple resources, despite their specificity (Tuda et al. 2005). This aptitude allows them to remain in the forest, where multiple resources are available, explaining the occurrence of Diabrotica and Phyllophaga in the secondary forest.
We used several types of bait to understand the affinity of beetles to a particular type of food, which tends to be associated with their ecological roles and differs between taxa (Selfa & Anento 1997, Guseva & Koval 2013, Honek et al. 2013; Luna, 2015); by doing so we aimed to ensure that beetles from multiple guilds were well represented in croplands and in the secondary forest (Bogoni & Hernandez 2014). Our analysis showed coprophagous beetles as the dominant group, followed by saprophagous and necrophagous beetles. Coprophagous beetles are considered beneficial since they fulfill a cleaning function in forest areas where large amounts of excrement need to be processed to return to the soil and improve its fertility and aeration (Luzuriaga 2013). We report only one saprophagous exclusive morphospecies, Nitidulidae gen. sp1, which was found only in the secondary forest. This group feeds on decaying plant and organic matter, such as the bark of trees and decaying fruits (Pedraza et al. 2010), justifying its occurrence in the forest. However, it has also been reported as a post-harvest pest in strawberry fields (Gutiérrez et al. 2020).
During manual collections we found specimens of the families Erotylidae, Passalidae and the genus Pelidnota (Scarabaeidae) in decomposing organic matter that are also considered saprophagous (Arnett et al. 2002). Some morphospecies were found in all bait types, such as Canthidium sp1 and Canthon sp1 in the forest and Cratosomus sp1 and Phyllophaga sp1 in crops. The genera Canthidium and Canthon have been considered generalists since they utilize various substrates when there is low availability of their main resource (Dominguez 2012, Sulca & Huamantinco 2016); in contrast, Cratosomus and Phyllophaga are considered harmful pests common in croplands (Isabeth et al. 2003, Pérez et al. 2008).
We also collected specimens of the following genera: Macrodontia, Neoptychodes, Stizocera, Trichocnemis, Anomala, Conoderus (Eschscholtz 1829), Orthostethus, and Elateridae gen. sp1 and sp2. These groups use a wide spectrum of food sources, including live and decaying wood, seeds, and roots (Martínez 2000, Paucar-Cabrera 2005, Zurita-García et al. 2014). Mycophagous morphospecies (Mylaris sp1, Mycetophagidae, gen. sp1, Trogossitidae, gen. sp1 and Phileurus sp1) (Arnette et al. 2002) were also found.
Molina-Moreira & Arias (2019) state the need to carry out entomofauna studies in other dry forests on the Ecuadorian coast that will allow for comparisons between populations located within the forest and populations in fragmented areas. This study is the first to characterize the coleopteran community in the tropical dry forest of Manabí, reporting a significant diversity and representativity of 15 families including 64 morphospecies. This lays a foundation for future research on integrated pest management, community ecology, and disturbance assessment along the fragmented landscape of the Ecuadorian dry forest. According to Viana (1993), regenerated and remnant forests serve as refuges for wildlife that were once widely distributed in the area. Based on our initial analysis of beetle communities in regenerated forests, we suggest these patches should be protected for conservation purposes, and to prevent the possibility of insects becoming crop pests. While our work contributes valuable insights into the morphospecies composition of these forests, more detailed identification is necessary to fully understand the dynamics of the beetle community in these disturbed ecosystems, and to expand the checklist of beetle species in the Ecuadorian dry forest. Overall, these findings highlight the importance of preserving regenerated forests and continuing research efforts to better understand their ecological significance.












 uBio
uBio