Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Peruana de Biología
On-line version ISSN 1727-9933
Rev. peru biol. vol.18 no.2 Lima Aug. 2011
TRABAJOS ORIGINALES
Activity of ethanolic extracts leaves of Machaerium floribundum against acne-inducing bacteria, and their cytoprotective and antioxidant effects on fibroblast
Actividad del extracto etanólico de las hojas de Machaerium floribundum contra bacterias que inducen el acné y su efecto citoprotector y antioxidante sobre fibroblastos
Lorena Díaz1*
; Soumi De Montijo2 ; Ana L. Medina3 ; Pablo Meléndez1 ; Vian Laurence4 and Gilberte Marti-Mestres41 Department of Pharmacognosy and Organic Medications
2 Department of Parasitology and Microbiology
3 Department of Food Sciences
*Mail Lorena Díaz: loredive@yahoo.com
4IBMM, UMR5247, Facultad de Farmacia, Universidad Montpellier 1, 14491, 34093, Montpellier, Francia.
Abstract
Propionibacterium acnes, Staphylococcus epidermidis and Staphylococcus aureus have been recognized as the bacteria that are involved in the inflammatory process of acne, while oxidants and antioxidants are involved in the repair of cutaneous tissue affected. In this study an evaluation was made of the antibacterial effect by the agar diffusion and broth dilution method, the cytoprotective and antioxidant effect on 3T3 dermic fibroblast cells, treated with hydrogen peroxide and the scavenging capacity of free radicals was determined by the 2, 2-diphenyl-l-picrylhydrazyl (DPPH) method as well as the Reducing Power of the ethanolic extracts of the leaves of the Machaerium floribundum. Minimal bactericidal concentrations (MBC) were obtained against Propionibacterium acnes and Staphylococcus aureus of 5 mg/mL and 2 mg/mL, respectively. A cytoprotective effect of 111% was observed over the cellular viability of the fibroblasts at 10 μg/mL and an antioxidant effect of 92% over the viability of the fibroblasts treated with hydrogen peroxide at 25 μg/mL. A stimulation of 24% growth of fibroblasts at 50 μg/mL was evidenced. On the other hand a 93% scavenging activity of the DPPH free radical was shown for 100 μg/mL with a CI50 of 34 μg/mL. The reducing power was evidenced to be dependent on the concentration. The results obtained indicated that the ethanolic extract of Machaerium floribundum shows a good antibacterial activity against bacteria that induce acne and a high potential for scavenging of free radicals at relatively low concentrations.
Keywords: Machaerium floribundum; Acné; Antibacterial; Antioxidant; Fibroblasts.
Resumen
Propionibacterium acnes, Staphylococcus epidermidis y Staphylococcus aureus han sido reconocidas como las bacterias involucradas en el proceso inflamatorio del acné, mientras que oxidantes y antioxidantes han sido implicados en la reparación del tejido cutáneo afectado. El presente estudio evaluó el efecto antibacteriano por el método de difusión en agar y dilución en caldo; el efecto citoprotector y antioxidante sobre células de fibroblastos dérmicos 3T3, tratadas con peróxido de hidrogeno; se determinó la capacidad secuestrante de radicales libres por el método del 2,2-difenil-2-picrihidracil (DPPH) y el poder reductor de los extractos etanólicos de las hojas de Machaerium floribundum. El extracto mostro una CMB de 5mg/mL y 2mg/mL para P. acnes y S. aureus, respectivamente. Se observó un efecto citoprotector sobre la viabilidad celular de los fibroblastos de 111% a 10 μg/mL y antioxidante mostrado sobre la viabilidad de los fibroblastos tratados con peróxido de hidrogeno de 92% a 25 μg/mL. Se evidencio estimulación del crecimiento de fibroblastos de 24% a 50 μg/mL. Por otra parte se mostró actividad secuestrante del radical libre DPPH de 93% a 100 μg/mL, con una CI50 34 μg/ mL. El poder reductor evidencio ser dependiente de la concentración. Los resultados indicaron que el extracto etanólico de Machaerium floribundum presenta una buena actividad antibacteriana contra las bacterias que inducen el acné y un alto potencial secuestrante de radicales libres a concentraciones relativamente bajas.
Palabras claves: Machaerium floribundum; Acné; Antibacteriano; Antioxidante; Fibroblastos.
Introduction
Machaerium species consist of lianas, bushes and trees found from sea level up to 500 – 900 m, and rarely above 1,700 m, it is distributed from Mexico to Argentina (Lozano et al. 2006), and 39 taxa are reported for Venezuela (Meléndez 2009). In the traditional medicine of Peru, Machaerium species have been used for the treatment of diarrhea and sexual impotency (Rengifo 2001). The procyanidin obtained from the ethanol extract of ligneous stems and bark of M. floribundum showed antibacterial activity against Pseudomonas maltophilia and Enterobacter cloacae (Waage et al. 1984). No other activity has been reported for this species.
Acne vulgaris is a common illness that affects the areas of the body that have big sebaceous glands such as the face, back and trunk (Leydon 1997). The normal bacterial flora of the skin includes Propionibacterium acnes, Staphylococcus epidermidis, S. aureus and Pityrosporum ovale, which proliferate during puberty and often are involved in the development of acne (Hamnerius 1996). Propionibacterium acnes has been described as an inflammatory anaerobic organism that is implicated in the development of inflammatory acne, while S. epidermidis and S. aureus are aerobic organisms that usually are involved with superficial infections of the sebaceous unit (Burkhart et al. 1999). For many years antibiotics have been used for the treatment of acne. However, resistance to antibiotics has increased and in a multifactorial manner, which includes the bacteria-antibiotic relationship, the type of antibacterial, and the characteristics of the host, among others. To overcome the problem of resistance to antibiotics, medicinal plants have been studied extensively as alternative treatments.
The cutaneous aging process, whether physiological or as a consequence of other exogenous factors, is always related at the molecular level with the appearance of non-controlled oxidative activities. Thus, cellular catabolism takes place through the oxidative process of the Krebs cycle. This process is responsible for the generation of H2O2 in the interior of cutaneous cells.
Likewise, the oxidative reactions that are not of enzymatic origin require the presence of oxygen. The most frequent reaction is linked to the energetic contribution of UV photons, which are captured by chromophore molecules present in the cutaneous tissue and are conducive to the transfer of an electron to the oxygen molecule and the formation of very reactive oxygen species (ROS) (Parra et al. 1995a). The cells of mammals have an elaborate defense mechanism for detoxification of free radicals, such as the enzymes dismutase superoxide (SOD), catalase (CAT), and glutathione peroxidase (GPX). Besides these, there are several antioxidant molecules that play an important role in the antioxidant defense system. These molecules can be synthesized either in vivo, for example glutathione, bilirubin and melatonin, or can be obtained from the diet, such as vitamins (α-tocopherol, β-carotene and ascorbic acid) and micronutrients, such as zinc and selenium (Si Eun et al. 2003).The imbalance between the production of free radicals (ROS) and the quantity of antioxidants available gives place to oxidative stress. This can cause damage to cells and tissues during infections, as well as several degenerative disorders, such as cardiovascular, cell aging and neurodegenerative conditions, like Alzheimers disease, mutations and cancer, (Ames 1998). Currently, a great variety of plant extracts are used for their antioxidant potential, for their stimulation of growth, and their cytoprotective effect on fibroblasts (Si Eun et al. 2003, Annan & PHoughton 2008, Phan et al. 2001). In this study, the activity of ethanol extracts of the leaves of M. floribundum against acne-inducing bacteria was determined, as well as their cytoprotective and antioxidant activity against 3T3 dermic fibroblast cells. This work was conducted with the aim of finding an alternative treatment for acne vulgaris and the regeneration of cutaneous tissue.
Material and methods
Chemicals
Modified Eagles Medium (DMEM) with 4.5 g/L of glucose, BioWhitaker®, provided by Lonza and supplemented with 10% fetal bovine serum (FBS), 1.2% of a mixture of penicillin and streptomycin (P/S), and 1.2% of L-glutamine (Glu). Neutral red colorant (3-amino-7-dimethylamino-2-methylfuroside hydrochloride), 2,2-diphenyl-l-picrylhydrazyl (DPPH), potassium ferrocyanide (K3Fe(CN)6), ferric chloride (Cl3Fe), ascorbic acid, hydrogen peroxide (H2O2) and catalase of bovine liver were provided by Sigma-Aldrich (France).
Microorganisms
Propionibacterium acnes (CVCM 1453), Staphylococcus aureus (CVCM 764), and S. epidermidis (CVCM 352) were provided by the Venezuelan Collection of Microbiology Culture, Venezuela.
Collection and extraction of the plant
Machaerium floribundum Benth. was collected in the Caparo Forrest Reserve in the state of Barinas, Venezuela. A sample of the plant identified with the number 562 (collection P. Melendez and R. Nuñez) was deposited in the Herbarium of the Faculty of Pharmacy and Bioanalysis (MERF) of the Universidad de Los Andes, in Mérida, Venezuela.
The plant material was oven dried at 40 ºC for 72 h and then powdered. A sample (100 g) was extracted with 500 mL ethanol at room temperature in the dark for 8 days, filtered through a Whatman Nº 1 filter paper, and the filtrate dried in a rotary evaporator at 45 ºC. The dry extract was stored at 7 ºC.
Antibacterial activity
A 1.5 x 108 UFC/mL bacterial inoculum was prepared from each of the strains according to the 0.5 Mc Farland pattern (NCCLS 2003).
Diffusion method in agar.- This trial was performed by the method of Hayes and Markovic (2002), with some modifications. A solution of the ethanolic extract was prepared at a concentration of 100 mg/mL in ethanol. Previously sterilized plates were prepared by the addition of 15 mL of Muller Hinton agar (agar base), which was allowed to solidify. Using sterile forceps, stainless steel cylinders of 7 mm diameter were placed over the agar. Later, 5 mL of the same agar, previously inoculated with 100
μl of the standardized bacterial inoculum (1,5 x 108 UFC/ mL), was added; this was allowed to solidify and the rings were withdrawn, thus leaving the agar with holes. In the resulting reservoir, 20 μL of ethanolic extract was added, as well as negative (ethanol) and positive controls (ampicillin at 10 μg/mL). After 30 minutes, the plates were incubated under either anaerobic or aerobic conditions, according to the bacteria tested. The results were determined by measurement in millimeters the inhibition zones and comparison of these with the inhibition zone produced by ampicillin; all results are an average of three trials.Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC).- This trial was carried out according to the method of Kumar et al. (2007), with some modifications the inoculum was standardized by the method described above (Hayes and Markovic 2002). The extracts were tested at different concentrations (5, 2.5, 2, 1.5 and 1.25 mg/ mL). Since the strains withstand 5% ethanol at the maximum, stock solutions were prepared at 100, 50, 40, 30, and 25 mg/ mL. Four series of six sterilized tubes (two series per bacterial species) were dosed with 1.9 mL of glucose-yeast broth for the Staphylococcus and the same medium supplemented with 1% glucose for the Proponibacterium, 100
μL of extract, one for each concentration, and they were inoculated with 15 μL of the bacterial suspension standardized (1.5 x 108 UFC/mL), including the controls (bacterial growth, solvent, and sterility of the extract). The tubes were incubated for 24 hours at 37 ºC in aerobic conditions for Staphylococcus aureus, and for 48 hours at 37ºC for Propionibacterium acnes in anaerobic conditions, with gas pack envelopes. Measurement for percentage transmittance was taken at 625 nm before and after incubation in order to determine the MIC, with this being the lowest concentration of the extract that inhibits the visible growth of the microorganism. In this case, in which the extracts are colored, it is the lowest concentration at which no change in the percentage transmittance reading is verified. Once the time had elapsed, 15 mL of glucose-yeast agar was inoculated with 5 μL of each culture obtained in the prior phase and added to the Petri dishes. These were incubated under the same conditions as above. The UFC/mL count of each plate was made, with the MBC being the lowest concentration of the extract that completely inhibits bacterial growth.Cytoprotector Activity
Cellular proliferation of 3T3 fibroblasts.-
3T3 dermic fibroblast cells were obtained from the Medications Toxicology Laboratory of the Faculty of Pharmacy Montpellier 1 in France. Cells in confluent growth were trypsinized, centrifuged and resuspended in Modified Eagles Medium (DMEM) with 4.5 g/L of glucose, supplemented with 10% fetal bovine serum (FBS), 1.2% of a mixture of penicillin and streptomycin (P/S), and 1.2% L-glutamine (Glu). The cells were counted in a CASSY.1 Brand hemocytometer and the cell concentration was standardized at 1x105 cells/mL in the same medium. The cells were dosed to a density of 1 x 104 cells/mL per well in plates of 96 wells. The plates were maintained at 37 °C for 24 hours in an incubator with 5% CO2, then the cells were lavaged with 0.0095M (PO4) Dulbeccos phosphate buffer saline (DPBS) without calcium and magnesium. Later, the extracts prepared in the DMEM culture medium were added at concentrations of 16.6, 31.25, 62.5, 125 and 250 μg/mL, with DMEM medium in 0.5% ethanol, as positive control, being placed in the first column (NICEATM 2003). After 24 hours of incubation, cellular viability was measured by the neutral red test.Neutral Red Test.- After 24 hours of incubation of the aforementioned culture, the medium was discarded and the cells lavaged two times with 150
μL of DPBS. Then, 150 μL of a 1.25% solution of neutral red in DMEM culture medium was added. The cells were incubated for 3 hours at 37 °C in 5% CO2 before the neutral red solution was discarded and the cells lavaged two times with DPBS buffer. Later, 150 μL of a developing solution prepared with water: ethanol: acetic acid (49%:50%:1%) was added and the absorbance measured at 540 nm using an Elisa Bio-Rad reader (NIEHS 2003).Stimulation of growth of 3T3 fibroblast cells.- This trial used the method of Annan and Houghton (2008), with some modifications. Fibroblast cells in confluent growth were trypsinized, centrifuged and resuspended in Modified Eagles Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% glutamine, 53 units of penicillin and 53 mg/mL of streptomycin. The cells were counted in a CASSY 1 Brand hemocytometer and the concentration was standardized to a concentration of 1x105 cells/mL in the same medium. Using a multichannel micropipette the cells were dosed to a density of 1 x 104 cells/mL per well in plates of 96 wells. The plates were maintained at 37 ºC for 24 hours in an incubator with 5% CO2 before the cells were washed with 0.0095M (PO4) Dulbeccos phosphate buffer saline (DPBS) without either calcium or magnesium. Later, 100
μL of the extracts prepared were added at concentrations of 25, 50, 100, and 250 μg/mL in DMEM, supplemented with 0,5% fetal bovine serum (FBS), 1% glutamine, 53 units of penicillin and 53 mg/mL of streptomycin (6 mL of a mixture of penicillin, streptomycin (P/S) and 6 mL of glutamine). DMEM medium, 10% FBS, and DMEM medium with 0.5% FBS were used as positive control. The plates were maintained at 37 ºC for 72 hours in an incubator with 5% CO2 and the test was developed with neutral red.Antioxidant Activity
DPPH free radical scavenging activity.- Ethanolic extracts (200
μL) at concentrations of 25, 50, 100, 200, 250, and 500 μg/mL were added to 2.8 mL of a solution of DPPH at 6 x 10-2 mM prepared in methanol. Ascorbic acid was used as a positive control at a concentration of 176 μg/mL. The reaction mixtures were incubated in darkness for 30 minutes. Later, the absorbance was measured at 517 nm with a UV Kontron spectrophotometer.The inhibition percentage (%IP) of the DPPH free radical was calculated:
%IP = [Abs DPPH – Abs sample/Abs DPPH] x 100
The concentration required to obtain 50% of the maximum capacity to capture free radicals (CI50) was calculated by the following equation:
ΔCI50 = C1 – ΔC
C = [(ΔC1 – C2) x PI1 – 50]/ (PI1 – PI2)
PI1 and PI2: inhibition percentage immediately higher and lower than 50% of inhibition respectively.
ΔC1 – C2: concentrations at which IP1 and IP2 are produced respectively (Murillo et al. 2007, Goupy et al. 1999).
Assessment by reduction of metal ion.- One milliliters of extract, prepared at concentrations of 20, 50, 100, 125, 250, and 500 μg/mL, was mixed with 2 mL of phosphate buffer (0.2 M at 6.6 pH) and 2 mL of potassium ferricyanide [K3Fe(CN)6, 10g/L (1%)]. The mixture was incubated for 30 minutes at 50 ºC before 2 mL of 10% trichloroacetic acid was added and the mixture centrifuged at 3000 rpm for 10 minutes. Finally, 2 mL of the supernatant solution was mixed with 2 mL distilled water and 0.5 mL ferric chloride (0.1%), and the absorbance measured at 700 nm in a Genesis brand spectrophotometer. The increase in absorbance of the mixture indicated an increase in the reducing power. Ascorbic acid was used as reference at 5, 15 and 30 μg/mL concentrations (Ali et al. 2003, Palanisamy et al. 2008).
Antioxidant capacity in cultured 3T3 dermic fibroblast cells.- Annan and Houghtons (2008) method was used, with some modifications. The culture of 3T3 fibroblast cells was made as indicated in the cytotoxicity test. From the cellular suspension of 1x105 cells/mL, 100 μl (1x104 cells) was dosed in each well of a plate with 96 wells. The plates were kept at 37 ºC for 48 hours in an incubator with 5% of CO2, until the formation of a confluent monolayer of cells. Then, the cells were washed with 150 μL of DPBS phosphate buffer. Later, 100 μL of a mixture of the extracts at concentrations of 25, 50 , 100 and 250 μl/mL, and hydrogen peroxide (1x10-3 M) prepared in a DMEM culture medium, were added. The plates were kept at 37 ºC for 3 h, in an incubator with 5% CO2 before the medium was discarded and the cells washed with 150 μL of DPBS buffer solution. Catalase, at 250 Units/mL, was used as positive control. Cellular viability was determined by the neutral red test (NIEHS 2003).
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) of six replications for the assays on fibroblast cells and three replications for the other tests. One-way analysis of variance (ANOVA) was used, preceded by Cochran's tests (to check variances homogeneity), and followed by the Newman–Keuls multiple comparison test; significance was established at p<0.05.
Results and discussion
Antibacterial activity
Due to the high incidence of resistance of the bacterial strains that induce acne (Swanson 2003), there is a continuous demand for new therapeutic agents. In many countries, approximately 80% of the drugs available come from medicinal plants whose active principles, once elucidated, can be obtained by chemical synthesis (Penso 1980). In Table 1, a strong inhibitory effect is shown on the growth of the studied strains, and inhibition zones ≥ 15 mm are observed compared with those produced by 10
μg/mL ampicillin. Staphylococcus aureus showed the greatest sensitivity to the extract.
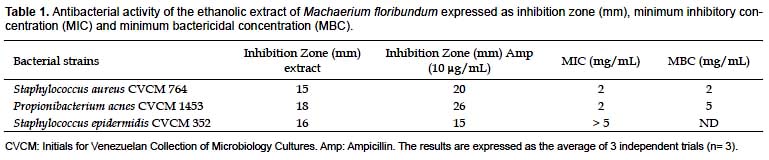
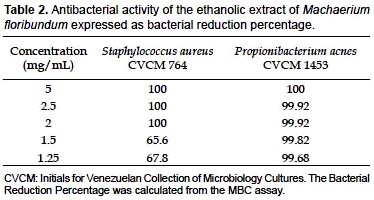
The MIC and MBC against S. aureus were both 2 mg/mL, while against Propionibacterium acnes they were 2 and 5 mg/mL, respectively (Table 1). However, for the latter species, a bacterial reduction percentage of 99.92% at 2 mg/mL was verified (Table 2), which suggests that the ethanolic extract of M. floribundum can act as a bactericidal agent against those microorganisms. Since these bacteria can tolerate only 5% ethanol and the MIC found against S. epidermidis was > 5 mg/mL, the MBC was not determined for this latter one. Nonetheless the extract showed good antibacterial properties against S. epidermidis through the agar diffusion method in which an inhibition zone of 16 mm was obtained compared with 15 mm for ampicillin at 10
μg/ mL. The control, 5% ethanol, did not inhibit bacterial growth compared with that of each bacterial strain without 200 μL ethanol; while the ampicillin positive control inhibited bacterial growth at 10 μg/mL. It should be noted that, according to Fabry et al (1998), for a crude extract of a plant to be considered potentially useful therapeutically, it must have an MIC value < 8 mg/mL. The ethanolic extract M. floribundum leaves showed lower MIC and MBC values.
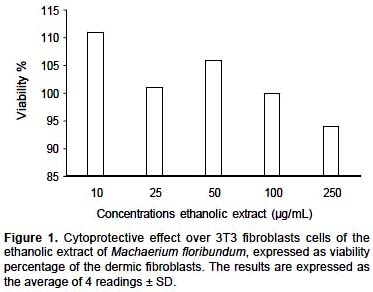
Cytoprotector Activity
Effect on cell viability of 3T3 dermic fibroblasts.- The cytoprotective activity of the ethanolic extract of M. floribundum (Fig. 1) on the growth of dermic fibroblasts (3T3) was not dose dependent and a cellular viability percentage greater than 100% ± 6.9% at 10, 25 and 50
μg/mL was observed. The cellular viability began to decrease at 250 μg/mL, for which a viability percentage of 94% ± 7% was obtained. The ethanolic extract of M. floribundum showed good cytoprotecive activity over the growth of 3T3 dermic fibroblasts up to 100 μg/mL.
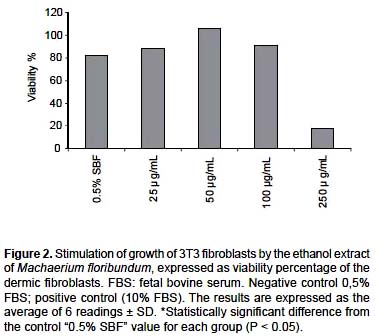
Stimulation of growth.- The proliferation and stimulation of growth of fibroblasts is important in tissue repair since the fibroblasts are involved in the migration, proliferation, contraction and production of collagen (Woodley et al. 1985, Mimura et al. 2004). The ability to stimulate the growth of fibroblasts is now considered to be a model to evaluate the in vitro activity over the healing process of wounds (Mensah et al. 2001, Houghton et al. 2005). In Figure 2, the effect of the ethanolic extract of M. floribundum on the stimulation of growth of 3T3 fibroblasts is shown. At 50 and 100
μg/mL, a significant increase (P < 0.05) on cellular growth of 24 % ± 5,1% and 9% ± 2,6% was observed compared with the control, in which the cells grew with minimum growth factors and without the extract. Statistical analysis indicates that 25 and 100 μg/mL are homogeneous groups (P < 0.05). At 250 μg/mL, toxicity of the extract on the cells was observed, even though such toxicity was not seen in the prior assay (Effect on cell viability) in which cellular growth was verified with the maximum growth factors.
Antioxidant Activity.
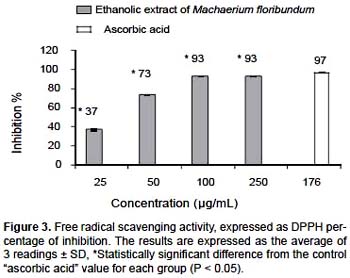
DPPH free radical scavenging activity.- Using the DPPH test, the ethanolic extract of M. floribundum revealed a significant antioxidant activity (P < 0,05) with a CI50 of 34
μg/mL. In Figure 3, an activity dependent on concentration is evidenced up to 100 μg/mL, with an inhibition percentage of 93% ± 0,1%, compared with 97% ± 0,1% obtained for ascorbic acid, the positive control, at a concentration of 176 μg/mL. On this basis it can be said that the ethanolic extract of M. floribundum leaves has great potential for the prevention and treatment of the damage induced by the imbalance of the ROS at the organic level.
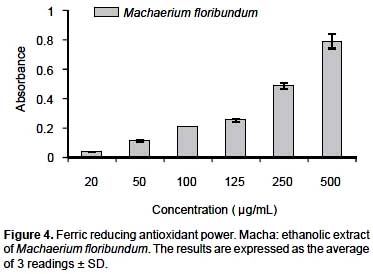
Antioxidant assessment by reduction of metal ion.- The antioxidant activity of the plant extract was complemented by measuring its capacity to reduce Fe+3 to Fe+2, monitored by the formation of a colored complex (Fenton type reaction). Figure 4 illustrates the reducing power of the ethanol extract at 25, 50, 100, 120, 250 and 500
μg/mL compared with ascorbic acid, a positive control, at concentrations of 5, 15 and 30 μg/mL. It was evidenced that the reducing power of the extract and of the control were dependent on the concentration, taking note of an absorbance of 0.79 nm ± 0.05 for M. floribundum at 500 μg/ mL. These results show that the extracts have a high potential antioxidant capacity at relatively low concentrations.
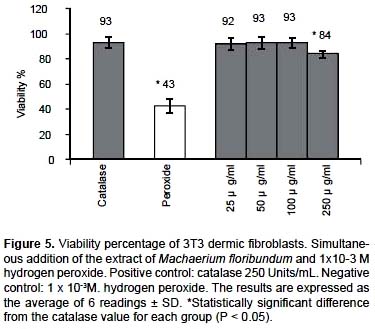
Antioxidant capacity in cultured 3T3 dermic fibroblast cells.- It is well known that hydrogen peroxide causes oxidative damage to cells. In Figure 5, it is shown that the ethanol extract of M. floribundum at 50
μg/mL revealed a viability of 93% ± 4.9% of the fibroblasts compared with the same percentage for 250 units/mL of catalase, used as a positive control, and of 43% ± 5.5% for 1x10-3 M hydrogen peroxide. A significant percentage of 50% protection was verified (P < 0.05) over the cellular viability of the dermic fibroblasts for the ethanol extract of M. floribundum likewise, concentrations of 25, 50, and 100 μg/mL and catalase (93 % ± 4.2) are identified as homogenous groups, The most likely mechanism of the antioxidant effect is the direct interaction of the extracts with the hydrogen peroxide, more so than either the alteration or interaction of the extract with the membrane that can limit the damage induced by hydrogen peroxide (Annan et al.2008).
Conclusions
The regeneration of cutaneous tissue is characterized by re-epithelization and granulation of the tissue and remodeling of the extracellular array (Priya et al. 2002). The fibroblast cells play a very important role in these processes since they synthesize diverse proteic fibers (reticular, elastic and collagen) and the different macromolecules that are part of the fundamental substance (Parra et al. 1985b).
Oxidants and antioxidants are involved in the repair of cutaneous tissue. Oxidants contribute to tissue damage in the events following lesions of the skin, impairing the process of tissue regeneration. Antioxidants, on the contrary, prevent tissue damage and stimulate tissue recovery (Parra et al. 1995a). Research on the application of antioxidants of plant extracts for healing wounds has been widely published (Tran et al. 1997, Fronza et al. 2009).
The antioxidant properties of the ethanol extracts of the leaves of M. floribundum have been demonstrated scientifically in this research. It should be noted that it is the first report with regard to the effect of ethanol extracts of the leaves of this plant on cellular proliferation and antioxidant activity in dermic fibroblasts. Fractionation and purification studies are in progress to determine the active compounds and identify their chemical structures.
This research showed that the ethanol extract of the leaves of M. floribundum has good activity against bacteria that induce acne and, at 50
μg/mL, showed evidence of a very interesting scavenging activity of free radicals and cell proliferation for which its active components can be considered as an alternative for use in wound-healing.Acknowledgments
We thank the Collaboration Program between France and Venezuela (PCP) Food and Cutaneous Biosecurity and Consejo de Desarrollo Científico Humanístico, Tecnológico y Arte (CDCH-TA), Project Nº FA 440-08-03-B, of the Universidad de Los Andes in Mérida, Venezuela for financial assistance for this research.
Literature cited
Ali Y., M. Ahmet & A.K. Ayse. 2003. Antioxidant and antimicrobial activities of Polygonum cognatum Meissn extracts. Journal of the Science of Food and Agriculture. 83:64-69.
Ames B. 1998. Micronutrients prevent cancer and delay aging. Toxicology Letters 102:5-18.
Annan K. & P. Houghton. 2008. Antibacterial, antioxidant And fibroblast growth stimulation of aqueous extracts of Ficus asperifolia Miq. and Gossypium arboretum L Wound- Healing plants of Ghana. Journal of Ethnopharmacology.119:141-144.
Burkhart C.G., C. Burkhart & P.F. Lehmann. 1999. Acne: a review of immunologic and microbiologic factors. Journalof postgraduate Medicine. 75: 328-331.
Fabry W., P.Q. Okemo & R. Ansor. 1998. Antibacterial Activity of East African medicinal. Journal of Ethnopharmacology.60:79–84.
Fronza M., B. Heinzmann., M. Hamburger, et al. 2009. Determination of wound healing effect of Calendula Extracts using the scratch assay with 3T3 fibroblasts. Journal of Ethnopharmacology. doi:10.1016/j.jep.09.014
Goupy P., M. Hugues, P. Boivin et al.1999.Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. Journal of the Science of Food and Agriculture 79: 1625-1634.
Hamnerius N. 1996. Acne-etiology and pathogenesis. Treatment of Acne 32: 29-38.
Hayes A.J & B. Marcovic. 2002. Toxicity of Australian Essential oil Backhousia citriodora (Lemon myrtle). Part 1 Antimicrobial activity and in vitro cytotoxicity. Food And Chemical Toxicology 40: 535-543.
Houghton P.J., P.J. Hylands, A.Y. Mensah, et al. 2005. Effects of Buddleja globosa leaf and its constituents relevant to wound healing. Journal of Ethnopharmacology 100:100-107.
Kumar G.S., K.N. Jayaveera, C.K. Ashok, et al. 2007. Antimicrobial effects of Indian medicinal plants against acne-inducing bacteria. Tropical Journal of Pharmaceutical Research 6: 717-723.
Leydon J.J. 1997. Therapy for acne vulgaris. The New England Journal of Medicine 336:1156-1162.
Lozano P. & B. Klitgaard. 2006. The genus Machaerium (Leguminosae: Papilionoideae: Dalbergieae) in Ecuador.
Brittonia 58:124-150.Meléndez P. 2009. Sinopsis del Género Machaerium Pers.(Leguminosae-Papilionoideae-Dalbergieae) en Venezuela.
Acta Bot. Venez. 32:363-416.Mimura Y., M. Ihn., Y. Asano., et al. 2004.Epidermal Growth factor induces fibronectin expression in human Dermal fibroblasts via protein kinase C
δ- signaling pathway. Journal of Investigative Dermatology 122:1390-1398.Mensah A.Y., J. Sampson., P.J. Houghton.,et al. 2001.Effects of Buddleja globosa leaf and its constituents relevant to wound healing.
Journal of Ethnopharmacology 77:219-226.NCCLS. National Committee for Clinical Laboratory Standards: Performance Standards for Antimicrobial Disk Susceptibility Tests (Approved Standard, M2-A5). 1993. Villanova, PA, National Committee for Clinical Laboratory Standards.
NICEATM. Center for the Evaluation of Alternative Toxicological Methods.2003.Test Method Protocol for Solubility Determination. In vitro Cytotoxicity Validation Study. Phase III. Prepared by The National Toxicology Program (NTP) Interagency
NIEHS. Institute of Environmental Health Sciences.Department of Health and Human Services. 2003.Test Method Protocol for the NHK Neutral Red Uptake Cytotoxicity – Validation Study.Phase III National.
Murillo E., O. Lombo, M. Tique, et al. 2007. Potencial antioxidante de Bauhinia kalbreyeri Harms (Fabaceae).Informacion tecnologica.
18:65-74.Palanisamy M., W. Chi-Lin, C. Hui-Ting, et al. 2008. Antioxidant activity of the ethanolic extract from The bark of Chamaecyparis obtusa var. formosana. Journal of the Science of Food and Agriculture 88: 1400-1405.
Parra J.L & G. Pons. 1995a.
Cosmetica antienvejecimiento. In: Ciencia Cosmética. Bases fisiológicas y Criterios Prácticos. Consejo General de Colegios Oficiales Farmacéuticos, Editores. Madrid: 466-471.Parra J.L & G.L. Pons. 1995b. La piel y sus anejos como sustrato vivo de la cosmetología. In: Ciencia Cosmética. Bases fisiológicas y Criterios Prácticos. Consejo General De Colegios Oficiales Farmacéuticos, Editores.
Madrid: 1-83.Penso G. 1980. The role of WHO in the selection and characterization of medicinal plants. Journal of Ethopharmacology 2:183–188.
Phan T.T., P. See, S. Lee, et al. 2001. Anti-oxidant effects Of extracts from the leaves of Chromolaena odorata on Human dermal fibroblasts and epidermal keratinocytes Against hydrogen peroxide and hypoxanthine-xanthine oxidase induced damage. Burns 27: 319-327.
Priya K.S, A. Gnanamani, N. Radhakrishnan, et al. 2002. Healing potential of Datura alba on burn wounds in albino rats.
Journal of Ethnopharmacology 83: 193-199.Rengifo L. 2001. Programa de Aprovechamiento Sostenible de Biodiversidad. Sub proyecto: Plantas Medicinales y Biocidas de la Amazonia Peruana. Informe Técnico. Instituto de Investigaciones de la Amazonía Peruana, IIAP, Iquitos.
165pp. <http://www.iiap.org.pe/publicaciones/literatura gris/Plantas medicinales y biocidas.pdf> (Access 26/01/2011)Si Eun L., J.H Hyun, H. Jung-Sun, et al. 2003.
Screening of medicinal plant extracts for antioxidant activity. Life sciences 73:167-179.Swanson I.K. 2003. Antibiotic resistance of Propionibacterium acnes in Acnes vulgaris. Dermatology Nursing 5: 359–361.
Tran V.H., M.A. Hughes & G.W. Cherry. 1997. In vitro Studies on the oxidants and growth stimulatory activities of a polyphenolic extract from Cudrania cochichinensis used in the treatment of wounds in Vietnam. Wound Rep Reg. 5: 159-157.
Waage S., P Hedin & E Grimley. 1984. A biologically-active procyanidin from Machaerium floribundum.
Phytochemistry 23:2785-2787.Woodley D.T., E.J O´Keefe & M. Prunerias.
1985. Cutaneous wound healing: a model for cell-matrix interaction.Journal of the American Academy of Dermatology 12: 420-433.
Presentado: 26/01/2011
Aceptado: 27/05/2011
Publicado online: 25/08/2011













