Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Peruana de Biología
On-line version ISSN 1727-9933
Rev. peru biol. vol.18 no.2 Lima Aug. 2011
TRABAJOS ORIGINALES
Potential use of low-NDGA Larrea divaricata extracts as antioxidant in foods
Uso potencial de extractos de Larrea divaricata con bajo contenido de NDGA como antioxidantes en comidas
Sebastian Turner1
; Roberto Davicino1 ; Rosario Alonso1 ; Graciela Ferraro1,2 ; Rosana Filip1,2 and Claudia Anesini1,21 Institute of Chemical and Metabolism of Drugs- University of Buenos Aires- National Council of Scientific and Technical Research (IQUIMEFA-UBA-CONICET), Junín 956, University of Buenos Aires, Buenos Aires, Argentina.
2 Pharmacognosy Unit, Faculty of Pharmacy and Biochemistry, University of Buenos Aires, Junín 956, Buenos Aires, Argentina.
Abstract
Larrea divaricata Cav. is widely distributed in Argentina. Aqueous extract, of its leaves, has documented antitumoral and immunomodulatory activities. In this study, the antioxidant activity of aqueous extract and a component, nordihydroguaiaretic acid was determined and compared using different assays. Both the aqueous extract and nordihydroguaiaretic acid exhibited antioxidant activity. However, results show that it is very likely that compounds other than nordihydroguaiaretic acid could be involved in the antioxidant activity of the extract. Since nordihydroguaiaretic acid is nephrotoxic and hepatotoxic agent, it is important to direct efforts toward the potential use of low-nordihydroguaiaretic acid L. divaricata extracts as antioxidant in foods.
Keywords: Nordihydroguaiaretic acid; Larrea divaricata; antioxidant activity; superoxide dismutase; catalase
.
Resumen
Larrea divaricata Cav. está ampliamente distribuida en la Argentina. Se han documentado actividades antitumorales e inmunomoduladoras de los extractos acuosos de sus hojas. En este estudio, la actividad antioxidante del extracto acuoso y un componente, el ácido nordihidroguayarético, se determinaron y compararon mediante diferentes ensayos. Tanto el extracto acuoso como el ácido nordihidroguayarético mostraron actividad antioxidante. Sin embargo, los resultados muestran que es muy probable que otros compuestos diferentes al ácido nordihidroguayarético pudieran estar involucrados en la actividad antioxidante de los extractos. Dado que el ácido nordihidroguayarético es un agente nefrotóxico y hepatotóxico, es importante dirigir los esfuerzos hacia el uso potencial de extractos de L. divaricata con bajas cantidades de ácido nordihidroguayarético como antioxidantes en alimentos.
Palabras clave: Acido nordihidroguayarético, Larrea divaricata, actividad antioxidante, superóxido dismutasa, catalasa.
Introduction
Larrea divaricata Cav. (Zygophyllaceae) is distributed in the west of South America and widely in Argentina. It is used in folk medicine for the treatment of many diseases, due to its anti-inflammatory and anti-rheumatic properties (Ratera & Ratera 1980). The aqueous extract, of its leaves, possess well documented antitumoral and immunomodulatory activities (Anesini et al. 1996, Anesini et al. 2001), antimicrobial properties (Anesini & Perez 1993, Stege et al. 2006) and an antioxidant activity demonstrated on peroxidase secretion of rat salivary glands (Anesini et al. 2004). Larrea divaricata is botanically related with L. tridentata (Sesse and Moc. Ex DC) (creosote bush), a common shrub of North American warm deserts, both species present different geographical distribution but posses some compounds in common. Larrea tridentata has been introduced as a dietary supplement, mainly due to its antioxidant activity (Arteaga et al. 2005). Nordihydroguaiaretic acid (NDGA) which possesses antioxidant and inhibitory activity on inflammation mediators was isolated from L. divaricata leaves (Franchi-Micheli et al. 1986). Also, NDGA was used as an antioxidant food preservative for fats and butter, but it is no longer used for this purpose because of its toxicity (Yamamoto et al. 1970). Nevertheless, the antioxidant activity of the aqueous extract of L. divaricata previously reported on peroxidase secretion is not related with the presence of NDGA.
On the other hand, many antioxidant compounds naturally occurring in plant sources have been identified as free radical or active oxygen scavenger. Recently, there is great interest in detecting these antioxidant compounds for the potential use in foods in order to replace those synthetic antioxidants which possess carcinogenic effects (Gulzcin et al. 2004). In view of these facts, this is a first time that, a comparative study of the antioxidant activity of an aqueous extract of L. divaricata and NDGA was undertaken in order to evaluate the contribution of the latter to the antioxidant activity of the aqueous extract. Antioxidant properties were assayed using several methods, such as ferric thiocyanate (FTC), diphenylpicrylhydrazyl (DPPH) free radical scavenging methods, also, hydrogen peroxide scavenging-catalase (CAT) activity and oxygen scavenging -superoxide dismutase (SOD) activity was determined.
Material and methods
Drugs.- Linoleic acid, NDGA and stable radical DPPH were purchased from Sigma, San Diego, USA. Merck, Darmstadt, Germany solvents were used for chromatography. NDGA was dissolved in HPLC quality methanol (Sigma, San Diego, USA).
Plant material.- Leaves of Larrea divaricata Cav., were collected in the province of Cordoba, Argentina and identified using morphological, anatomical and histochemical analysis. A voucher specimen was deposited in the Museum of Pharmacobotany, School of Pharmacy and Biochemistry, University of Buenos Aires.
Extracts.- An aqueous extract of L. divaricata was prepared as follows: air-dried leaves (750 mg) were extracted for 10 min with boiling distilled water (10 mL), heated for a further 45 min at 56 °C with mechanical agitation and let to rest during 72 h at 5 °C. The resulting extract was filtered and taken to dryness using a rotary evaporator, yielding a residue of 200 mg. This residue was re-dissolved in distilled water for experimental purposes. In order to determine the NDGA content, three different concentrations of the aqueous extract (2.5, 7.5 and 22.5% p/v) were prepared by submitting the required amount of air-dried leaves to the process described above. These yielded residues of 202.4 mg, 840.9 mg and 1485 mg, respectively (Anesini et al. 2001).
Hydrogen peroxide scavenging activity: CAT activity.- The rate of decomposition of hydrogen peroxide can be enzimatically catalyzed by the presence of peroxidases such as catalase. In this case, catalase activity of aqueous extract and NDGA at different concentrations (0.01–1000
μg/mL and 0.001–10 μg/mL respectively) was assessed by following the rate of disappearance of the substrate with a spectrophotometer at 240 nm set in the kinetic mode. The incubation mixture was prepared diluting 0.1 mL of sample in phosphate buffer (0.05 M pH 7); 50 μL of hydrogen peroxide were added immediately before beginning absorbance determination (final concentration 0.02 M). The absorbance was recorded during 5 min and plotted versus time. The initial rate of disappearance of hydrogen peroxide (absorbance/minute) was calculated from the initial linear part of the curve (45 sec). An extinction coefficient of 0.0394 cm2/nmol for H2O2 was used to calculate it concentration. The catalase activity was then calculated using the following conversion: one unit of catalase is the amount of enzyme required to decompose 1 mmol of hydrogen peroxide per min, at 25 °C and pH 7.0 (Carrillo et al. 1991). Results are expressed as U/mL.Oxygen reducing activity: SOD activity.- This assay is based on the property of compounds possessing a SOD activity to inhibit the spontaneous oxidation of adrenaline to adenochrome by reduction of oxygen. Adrenaline rapidly undergoes auto-oxidation at pH 10, 7 producing adrenochrome, which is a pink colored product that can be measured at 480 nm with UV/VIS spectrophotometry. It is possible, thus, to monitor SOD activity by monitoring the production of adenochrome with a spectrophotometer in kinetic mode. The incubation mixture was prepared by diluting 0.05 mL of aqueous extract and NDGA (0.01–1000
μg/mL and 0.001–10 μg/mL respectively) with sodium buffer phosphate (0.05 M pH 10.7); 0.05 mL of adrenaline (final concentration 2 mM) were added immediately before beginning absorbance determination. Results are expressed as units (U) of SOD activity/mL. One units of SOD activity induces approximately a 50% inhibition of the auto-oxidation of adrenaline (Carrillo et al. 1991).Antioxidant activity determination by the FTC method.- Antioxidant activity of the aqueous extract of L. divaricata and NDGA was determined using the FTC method. Linoleic acid can be oxidized forming peroxides. These can be detected by their reaction with FeCl2 and thyocyanate which consists in the oxidation by peroxides of Fe2+ to Fe3+, which in turn form a red-coloured complex with thiocyanate. The antioxidant properties of compounds can be evaluated by their inhibition of linoleic acid oxidation. A volume of 0.8 mL of different concentrations of the extract and NDGA were mixed with phosphate buffer (0.05 M, pH 7) and linoleic acid (2.5 % in ethanol) to obtain 4 mL of solution. The resulting solutions were incubated at 38.5 °C in a glass flask. Aliquots were removed at regular intervals and FeCl2/ thyocyanate solution was added in order to allow any peroxides resulting from the oxidation of linoleic acid to react forming the complex that can be detected spectrophotometrically at 500 nm. A Shimatzu UV 2101 PC scanning spectrophotometer was used for this purpose. This step was repeated every 24 h until the control reached its maximum absorbance value. High absorbance values therefore indicated high lino1eic acid emulsion oxidation. Solutions containing only phosphate buffer and linoleic acid were used as blanks. Data on total antioxidant activity are the average of results of duplicate samples performed by triplicate.
The percentage inhibition of lipid peroxidation of linoleleic acid was calculated applying the following equation: Inhibition of lipid peroxidation(%) = 100 – [(As/Ao) x 100], where Ao is the absorbance of the control reaction (linoleic acid alone) and As is the absorbance in the presence of the sample extract, NDGA or positive control of antioxidant activity (1 mg/mL ascorbic acid).
The EC50 values were calculated from data obtained graphically, using a mathematical method based upon the principle of a right-angled triangle: CE50= D – [(A – 50% max response). X]/Y, in which A is the immediately higher response of 50% max response; B is the immediately lower response of 50% max response; D= log concentration corresponding to A response; C= log concentration corresponding to B response; X= D – C; Y= A – B (Alexander et al. 1999).
Free radical DPPH scavenging activity.- Scavenging activities of aqueous extract and NDGA at different concentrations (0.01–1000
μg/mL and 0.001–10 μg/mL respectively) on the stable free radical DPPH were assayed using the modified Blois method (Blois 1958), by which the bleaching rate of DPPH is monitored at a characteristic wavelength in presence of the sample. A volume of 0.1 mL of aqueous extract of L. divaricata and solutions of NDGA in 70% ethanol were mixed with 0.5 mL of a 500 μM DPPH solution in absolute ethanol and 0.4 mL of a 0.1 M buffer of Tris-ClH, at pH 7.4. The absorbance was measured at 517 nm after 20 min of reaction in the darkness.The percentage decrease of DPPH was calculated by measuring the absorbance of the sample and applying the following equation: % of inhibition= [1 – (As/A0)] x 100, where As is absorbance of sample, i.e extracts, NDGA or positive control of antioxidant activity, A0= is the absorbance of the DPPH solution. Ascorbic acid solutions of different concentrations were used as positive controls for antioxidant activity.
HPLC analysis.- The HPLC analysis was performed in a Varian Pro Star instrument with UV photodiode array detector. A column C18 Gemini (150 mm x 4.6 mm and 5
μm ID) was used. The mobile phase was A: Water: acetic acid (98:2) B: Methanol: acetic acid (98:2); the gradient was from 15% B to 40% B in 30 min; 40% B to 75% B in 10 min; 75% B to 85% B in 5 min and 100% B in 5 min, leave 10 min 100% B and back to initial conditions. Flow 1.2 mL/min, room temperature. Detection: with UV 260 nm. The samples were analyzed with a program provided by Varian S.A (Filip et al. 2001).Animals treatment.- Seven week old male C3H/He mice were mainly provided by Dr. Norberto SanJuan (Dep. Microbiology. UBA) and maintained on a standard laboratory diet and water ad libitum. Animals were housed and cared for at the Animal Resource Facilities, Faculty of Pharmacy and Biochemistry, National University of Buenos Aires, in accordance with institutional guidelines. Mice were separated in several groups. One group of eleven animals was fed during 20 days with complete balanced feed (Cargill, Buenos Aires, Argentina). Second group of 20 animals was fed with complete balanced feed plus 33.4 mg/kg of aqueous extract in food. Third group containing eleven animals was fed with NDGA at 0.1 mg/kg that represents the approximate amount founded in extract (Davicino et al. 2006). After treatment animals were killed by cervical dislocation under anesthesia and blood was taken from the retro-ocular vein. The studies were approved by the institutional animal research committee, and were conducted in accordance with the internationally accepted principles for laboratory animal use and care (EEC Directive of 1986; 86/609/ EEC) (Rhiouani et al. 2008).
Toxicity studies.- Alanine transaminase (ALT) activity (IU/L) was determined in serum of extract/NDGA-treated or untreated mice using a commercial kit (Wiener Lab. Rosario. Argentina) (Davicino et al. 2007); cholesterol (mg%) and triglycerides (mg%) were evaluated by colorimetric methods and creatinine (mg/dL) was determined in serum of mice treated with aqueous extract or NDGA using a kinetic-colorimetric method.
Statistic analysis.- The data were recorded as mean value ± standard error of mean (SEM). One way analysis of variance was performed by ANOVA procedures. Significant differences between means were determined by Newman-Keuls test. P< 0.05 was regarded as significant.
Results and discussion
In the present study, an aqueous extract of L. divaricata and NDGA were investigated for their antioxidant activity using different methods. The results were then compared with the aim of analyzing the involvement of NDGA in the activity of the aqueous extract.
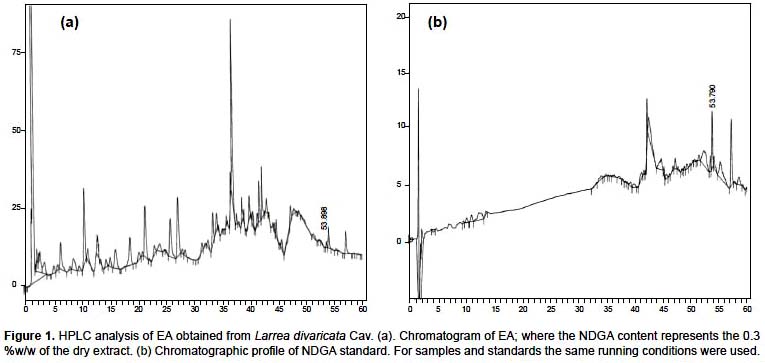
In the first place, the NDGA content of L. divaricata leaves was determined by HPLC, and represented 0.32 g %w/w (Figure 1 a). NDGA was identified by the retention time and UV spectral analysis of the peak against a standard of NDGA (retention time: 53.989 min similar to pure NDGA standard) (Figure 1b). The aqueous extract in a concentration of 1000
μg/ mL showed a concentration of 3.2 μg/mL of NDGA. Besides, we demonstrated that both, aqueous extract and NDGA at concentration found in the extract did not show toxic activity in mice (Table 1).
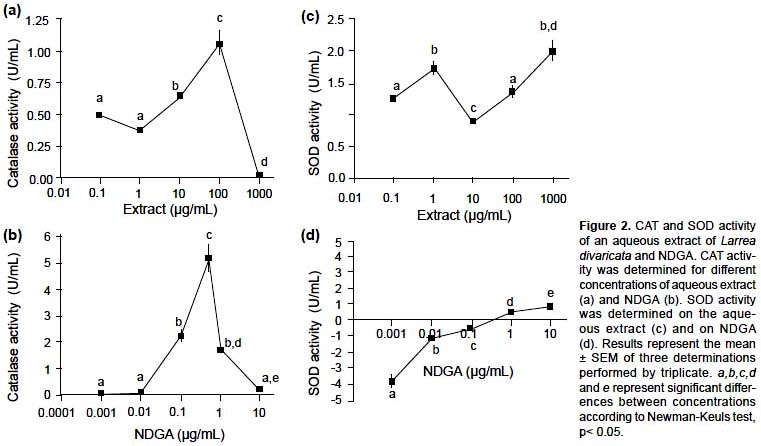
On the other hand, the extract exhibited catalase activity as is shown in Figure 2a with a maximum effect at 100
μg/ mL. The extract also presented SOD activity following two different patterns with maximum values at concentrations of 1 μg/mL and 1000 μg/mL (Figure 2c). In order to evaluate the contribution of NDGA to the antioxidant activity exerted by the extract, its catalase and SOD activities were determined. NDGA showed a greater CAT activity than the extract. CAT activity was exhibited at concentrations above 0.01 μg/mL with a maximum effect at 0.5 μg/mL (Figure 2b). A connection between CAT activity of the extract and the presence of NDGA could be established considering that maximum CAT activity of the extract appeared at a concentration of 100 μg/mL (NDGA content 0.32 μg/mL) and that the maximum exerted by NDGA was at 0.5 μg/mL. Besides, the extract appeared to have other antioxidant compounds apart from NDGA, which were responsible for the CAT activity exhibited at low concentrations of extracts. On the other hand, SOD activity of NDGA appeared but at concentrations above 1 μg/mL, while lower concentrations exerted pro-oxidative effect, revealed by negative values of SOD activity (Figure 2d). The extract exhibited SOD activity. This activity followed two patterns which depended on the concentration: from 0.1 to 10 μg/mL (A) and from 10 μg/ mL to 1000 μg/mL (B). The activity exerted by the extract at higher concentrations (B) could be related to the presence of NDGA, but as the activity of the extract was higher than that exerted by NDGA at these concentrations, others compounds with SOD activity could be present in the extract. The SOD activity observed at low concentrations of the extract (A), could be related to the presence of other antioxidant compounds. The pro-oxidant activity of NDGA has been proved by other authors, who observed this activity on a clone-9 rat hepatocyte culture, depending on concentrations and the biological environment (Sahu & Ruggles 2006).
The antioxidant activity on free radicals was also studied employing two different methods: a direct one, FTC, and an indirect one, DPPH. As in the other cases, different concentrations of L. divaricata aqueous extract and NDGA were tested. The FTC assay showed that the extract could exert a significant inhibition of linoleic acid peroxidation; although NDGA also presented inhibitory effects on linoleic acid peroxidation, the activity of the extract was higher than that exerted by NDGA (Figure 3a and 3b). It could therefore be inferred that NDGA was one of the active compounds present in the extract but that other antioxidants were also involved in this activity.
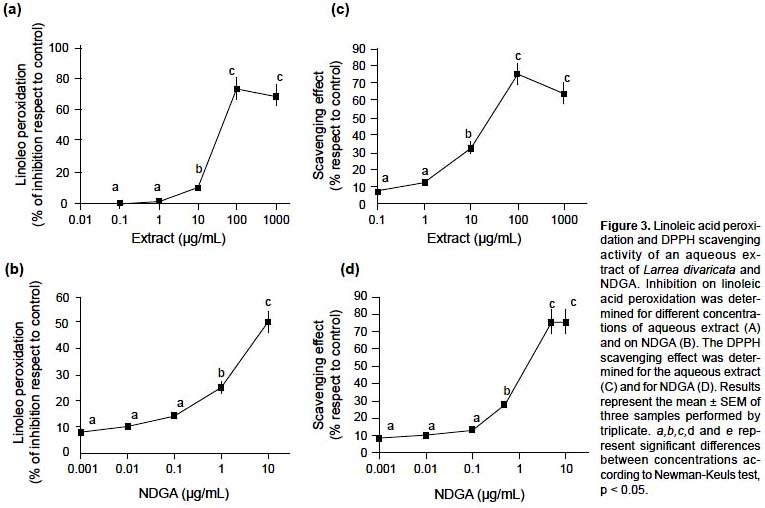
In the case of the DPPH assay, the extract displayed a significant DPPH radical scavenging activity in a concentration-response manner with EC50 of 13.18 ± 1
μg/mL (Figure 3c) related to the concentration. At EC50 of the extract the NDGA content was 0.042 μg/mL, meanwhile the EC50 of NDGA was 0.81 μg/mL (Figure 3d). Moreover at concentrations above 1 μg/mL the effect of the extract was greater than it exerted by the corresponding concentration of NDGA, meaning probably that at these concentrations of the extract, NDGA was not solely responsible for extract activity. At concentrations of extract below of 1 μg/mL, NDGA appeared to be the most important antioxidant compound. There is plenty evidence of the antioxidant activity of NDGA. The free radical scavenging activity of NDGA has been previously reported (Schreck et al. 1992) and was used as an antioxidant preservative for fats, rubber and butter (Arteaga et al. 2005, Yamamoto et al. 1970). Similarly, the antioxidant activity of a L. divaricata was also reported. Pedernera et al. (2006) detected principally DPPH scavenging activity in a methanolic extract.On the other hand, ascorbic acid, a known antioxidant agent, was used as positive control. For the FTC assay, ascorbic acid exerted 55 ± 5% of inhibition of linoleic acid peroxidation with respect to a control solution of linoleic acid. In the case of the DPPH assay, acid ascorbic also displayed a significant DPPH scavenging activity (% of inhibition respect to a control solution of DPPH): 0.1
μg/mL= 5 ± 0.4; 10 μg/mL= 65 ± 5; 100 μg/ mL: 75 ± 6; 1000 μg/mL: 82 ± 7 (data not shown).The chemical composition of L. divaricata has been studied previously. As well, the major components of the resinous coating of the plant are lignans among them nordihydroguaiaretic acid (NDGA). Moreover, in the leaves were found and identified flavonoids as non-watersoluble aglycones, water-soluble glycosides, and sulfated flavonoids (Mabry et al. 1977). The presence of saponins and leucoantocianidines was also reported (Bandoni et al. 1971). Among flavonoids were described: dihydrosyringetin, larragenine A, three O-glycosides from quercitin, myricetin, and a C glyoside from apigenin. Also were found, triterpenes, including sapogenins and monoterpenes like limonene (Mabry et al. 1977, Timmerman et al. 1979; Sakakibara et al. 1976, Bohnstedt and Mabry 1979).
It can be concluded therefore that NDGA is not the only compound with antioxidant activity present in the aqueous extract of L. divaricata. The extract has other compounds with probably even greater antioxidant activity depending on the type of assay performed. The flavonoids could contribute with both antioxidant and pro-oxidant activity, depending on their concentration (Sahu & Gray 1996). Flavonoids possess a remarkable spectrum of biochemical and pharmacological activities which can be attributed at least partially, to their antioxidant and free-radical scavenging properties (Middleton et al. 2000).
The antioxidant activities assayed in this study- especially radical scavenging activities- are very important due to the deleterious effect of free radicals on food and biological systems. It is known that, an accumulation of hydrogen peroxide can also be highly harmful, because it is formed in vivo and cross membranes, oxidizing a number of compounds. Considering the results of this investigation, since NDGA induces nephrotoxic and hepatotoxic effects (Arteaga et al. 2005), it is really important to find new natural antioxidant compounds from L. divaricata with low and non toxic amounts of NDGA.
The results obtained thus far, would demonstrate that the aqueous extract of L. divaricata clearly represent a promise as antioxidant substances with potential uses in foods.
Acknowledgement
This work was supported by UBACYT Grant B090 from Buenos Aires, University and CONICET Grant 5232 /05.
Literature cited
Alexander B., D.J. Browse, S.J. Reading, et al. A simple and accurate mathematical method for calculation of the EC50. Journal of Pharmacological and Toxicological Methods 41 (2-3): 55-58.
Anesini C. & C. Perez. 1993. Screening of plants used in Argentine folk medicine for antimicrobial activity. Journal of Ethnopharmacology 39 (2): 119-128.
Anesini C., A. Genaro, G. Cremaschi, et al. 1996. Immunomodulatory activity of Larrea divaricata. Fitoterapia 67: 329-334.
Anesini C., G. Ferraro, P. Lopez, et al. 2001. Different intracellular signals coupled to the antiproliferative action of aqueous crude extract from Larrea divaricata Cav. and nor-dihydroguaiaritic acid on lymphoma cell line. Phytomedicine 8: 1-7.
Anesini C., S. Turner, E. Borda, et al. 2004. Effect of Larrea divaricata Cav. extract and nordihydroguaiaretic acid upon peroxidase secretion in rat submandibulary glands. Pharmacological Research 49 (5): 441-448.
Arteaga S., A. Andrade-Cetto, et al. 2005. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. Journal of Ethnopharmacology 98 (3): 231-239.
Bandoni A.L., M.E. Mendiondo, V.D. Rondina, et al. 1971. Survey of Argentine medicinal plants I: folklore and phytochemical screening. Journal of Natural Products 35: 69-80.
Blois, M. S. 1958. Antioxidant determinations by the use of a stable free radical. Nature 26: 1199-1200.
Bohnstedt C. & T. Mabry. 1979. The volatile constituents of the genus Larrea (Zygophyllaceae).
Revista Latinoamericana de Química 10: 128-131.Carrillo M.C., S. Kanai, M. Nokubo, et al. 1991.
(–) Deprenyl induces activities of both superoxide dismutase and catalase but not glutathione peroxidase in the striatum of young male rats. Life Science 48 (6): 517-521.Davicino R., A. Mattar, Y. Casali, et al. 2006.
Activation and apoptosis of mouse peritoneal macrophages by extracts of Larrea divaricata Cav. (jarilla) International Immunopharmacology 6 (13-14): 2047–2056.Davicino, R., A. Mattar, Y. Casali, et al. 2007.
In vivo immunomodulatory effects of aqueous extracts of Larrea divaricata Cav. Immunopharmacology and Immunotoxicology 29 (3-4): 351 – 366.Filip R., P. López, G. Giberti, et al. 2001. Phenolic compounds in seven Southamerican Ilex species.
Fitoterapia 72 (7): 774-778.Franchi-Micheli S., S. Luzzi, M. Ciufti, et al. 1986.
The effects of lipoxygenase inhibitors and leukotriene antagonist on anaphylaxis, Agents Actions 18 (1-2): 242-244.Gulzcin I., V. Mshvidadze & A.R.E. Gepdiremen. 2004. Antioxidant activity of saponins isolated from ivy: alfa-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F.
Planta Medica 70 (6): 561-563.Mabry T.J., D.R. Difeo, M. Sakakibara, et al. 1977.
The natural products: chemistry of Larrea. In T.J. Mabry, J.H. Hunziker, D.R. Difeo, eds. Creosote Bush Biology and Chemistry of Larrea in New World Desserts, HutchinSon & Ross Inc., Dowden, Stroudsburg.Middleton Jr. E., C. Kandaswami & T.C. Theoharides. 2000. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacologicals Reviews 52 (4): 673-751.
Pedernera A. M., T. Guardia, C.G. Guardia Calderón, et al. 2006. Anti-ulcerogenic and anti-inflammatory activity of the methanolic extract of Larrea divaricada Cav. in rat.
Journal of Ethopharmacology 105 (3): 415-420.Ratera E. & M.O. Ratera. 1980. Plantas de la flora Argentina empleadas en medicina popular. Buenos Aires. Ed. Hemisferio Sur. 189 p.
Rhiouani H., J. El-Hilaly, Z.H. Israili, et al. 2008.
Acute and sub-chronic toxicity of an aqueous extract of the leaves of Herniaria glabra in rodents Journal of Ethnopharmacology 118 (3): 378–386.Sahu S.C., D.I. Ruggles & M.W. O`Donnell. 2006. Prooxidant activity and toxicity of nordihidroguaiaretic acid in clone-9 rat hepatocyte cultures, Food and Chemical Toxicology 44 (10): 1751-1757.
Sahu, S.C. & G.C. Gray. 1996. Pro-oxidant activity of flavonoids: effects on glutathione and glutathione S- tranferase in isolated rat liver nuclei, Cancer Letters 104 (2): 193-196.
Sakakibara M., D. Di Feo, N. Nakatami, et al. 1976.
Flavonoids methyl ethers on the external leaf of surface of Larrea divaricata and Larrea tridentate. Phytochemistry 15 (5): 727-731.Schreck R., K. Albermann & P.A. Bauerle. 1992. Nuclear factor kB: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radical Research Communications 17 (4): 221–237.
Stege P., R. Davicino, A. Vega, et al. 2006. Antimicrobial activity of aqueous extracts of Larrea divaricata Cav.
(Jarilla) against Helicobacter pylori. Phytomedicine 13 (9-10): 724-727.Timmerman B., A. Valesi & T. Mabry. 1979. Flavonoids from Larrea nitida, divaricata and cuneifolia. Revista Latinoamaricana de Química 10: 81-83.
Yamamoto K., M. Murata & H. Nakano.
1970. Antioxidant therapy, Larrea and livestock. Agricultural Biology and Chemistry 34: 1162-1168.
Correspondence to:
Dr. Roberto Davicino,
Immunology Unit, National University of San Luis,
Ejercito de los Andes 950, San Luis-Argentina.
Email Roberto Davicino: rcdavici@unsl.edu.ar
Email Sebastian Turner: sebaturner@hotmail.com
Email Rosario Alonso: mralonso@ffyb.uba.ar
Email Graciela Ferraro: gferraro@ffyb.uba.ar
Email Rosana Filip: rfilip@ffyb.uba.ar
Email Claudia Anesini: canesini@yahoo.com.ar
Presentado: 25/02/2011
Aceptado: 23/07/2010
Publicado online: 25/08/2011













