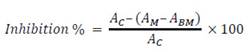Introduction
Macroalgae have been used as important source of highly nutritious food for thousands of years. They have also been used as fodder, fertilizer, and in the field of medicine, mainly in Asian countries (Kolanjinathan et al. 2014). They also produce bioactive compounds, including polyphenols, terpenoids, carotenoids, and tocopherols that possess antibacterial, antiviral, antifungal, antioxidant, anti-inflammatory, antitumor, and allelopathic properties (Michalak & Chojnacka, 2015). In addition, they are important because they have polysaccharides, called phycocolloids that are used in the food industry. These polysaccharides are the agar, carrageenan, and alginates obtained from some species of red (Rhodophyta) and brown (Phaeophyceae) algae.
In the Peruvian coast only few species of macroalgae are used. That is, some red algae species, such as Chondracanthus chamissoi “yuyo” and Pyropia sp. “cochayuyo” are used for food; Gracilariopsis lemaneiformis “pelillo” are exported for the agar industry; and the Phaeophyceae (brown algae) species, such as Macrocystis spp. “sargazo” and Lessonia spp. “aracanto” are exported for the alginate industry. However, Chlorophyta species (green algae) do not have a commercial interest yet because studies of their chemical compounds are scanty.
Caulerpa filiformis (Chlorophyta) was initially described by Howe (1914) for the north coast (Lobos de Afuera Island and Piura); however, later it was introduced in the central coast (Ancash, Lima, and Ica). Caulerpa filiformis is recognized as an invasive species for its rapid range expansion, colonizing new habitats such occurred in Paracas Bay (MINAM 2014; Ramsar 2015). In addition, its high productivity in places such as Paracas Bay causes a large amount of this algae to be stranded on the banks, where it accumulates and decomposes, generating bad odors, pollution, and a landscaping impact (personal observation), especially in spring (Pariona 2018).
Caulerpa have aroused interest worldwide for their secondary metabolites and for some activities useful for pharmaceutical industries, such as those that include antidiabetic (Sharma & Rhyu, 2014), antinociceptive and anti-inflammatory (Matta et al. 2015), antiviral (Nicoletti et al. 1999), antitumor (Cavas et al. 2006), antioxidant (Nguyen et al. 2011), antimicrobial (Vairappan, 2004), and anti-inflammatory (Stirk et al. 2003) properties.
The importance of knowing the antioxidant activity of C. filiformis is due to its possible potential as a natural source of antioxidants. Therefore, C. filiformis could be considered for use in the treatment of diseases, such as cancer, diabetes, and hypertension, whose pathophysiology is associated with the overproduction of reactive oxygen species (oxidative stress) (Leiva 2000). Currently, C. filiformis is not subject to any type of use, and studies on its biochemistry or pharmacological properties are scarce (Egg et al. 2015; Hernández et al. 2015). Therefore, the present study aims to generate knowledge about the main chemical groups (secondary metabolites) and the antioxidant properties of C. filiformis from Sechura Bay and Paracas Bay collected in the spring season where the highest biomass has been recorded.
Material and methods
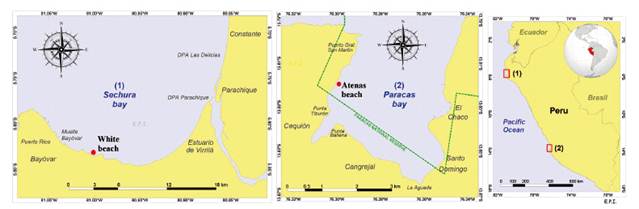
Algae collection.- First, C. filiformis was collected from the shallow submareal in two areas of the Peruvian coastline. Blanca Beach in Sechura Bay (5°49'50.8"S; 81°0'21.2"W) at 3 m depth and Atenas Beach in Paracas Bay (13°49′13.5″ S, 76°18′1.8″ W) at a 1.5 m depth in the spring season (September and October 2017, respectively). The distance among them are 1000 km approximately. Figure 1 shows the sites of collection. The samples were cleaned and washed in situ with seawater and transported to the laboratory, where they were immediately washed with potable water to remove excess sand and epiphytic organisms. They were then allowed to drain and dried at 40 °C.
Extract preparation.- The dry samples were subsequently pulverized, followed by the extraction of 5 g of this powder with 100 mL of 99.98% methanol at room temperature for 24 h on a magnetic stirrer. The mixture was centrifuged at 4500 rpm for 15 min at 10 °C, and the obtained supernatant was used in the experiment. The supernatants constituted the methanolic extracts of the C. filiformis composition (ES for Sechura Bay and EP for Paracas Bay) stored at −20 °C until use.
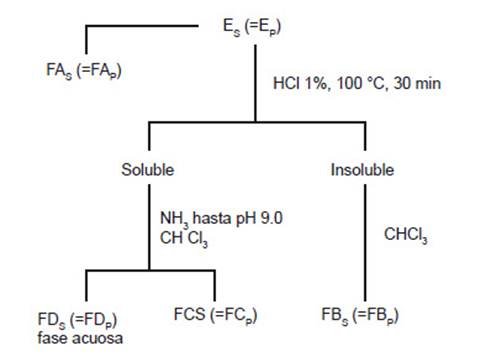
Preliminary Phytochemical Screening.- The methanolic extracts of C. filiformis from Sechura Bay (ES) and Paracas Bay (EP) were initially fractionated following the phytochemical guidelines (Rondina and Coussio, 1969) simplified in Figure 2.
The typical qualitative reactions of the coloration and the precipitation of the chemical groups were applied for the chemical analysis of the fractions obtained (i.e., FA, FB, FC, and FD).
Determination of the Total Phenol Content.- The total phenol content in the extracts of C. filiformis was determined according to the Folin-Ciocalteu test performed by López et al. (2011). Accordingly, 100 μL of the methanolic extract of C. filiformis (standard solution), 8.4 mL of distilled water, and 1 mL of an aqueous solution of Na2CO3 (20% w/v) were mixed. Subsequently, 500 μL of the Folin-Ciocalteu reagent was added. This mixture was kept stirred for 30 min at room temperature in the dark. The absorbance of the reaction mixture was measured at 765 nm using a spectrophotometer (Pharo300-Spectroquant). The results were expressed in mg of a gallic acid equivalent/g extract of the sample (mg AGE/g extract). The presented data corresponded to the average of three measurements.
Antioxidant activity.-
ABTS assay: The antioxidant activity of the methanolic extract of C. filiformis using the radical ABTS (2,2-azinobis-[3-ethylbenzothiazoline-6-sulfonic acid]) was determined according to the method adapted by Arnao et al. (2001). The ABTS radical (7.84 mg/mL) was previously activated with potassium persulfate (1.32 mg/mL) in the dark at room temperature for 12 h. The radical ABTS+ solution was obtained as a result of this reaction. This solution was diluted with 80% methanol until an absorbance of 1.1 ± 0.02 measured in a spectrophotometer at 734 nm. Next, 150 μL of the algae extract (standard solution) was reacted with 2,850 μL of the diluted solution of ABTS in a test tube. The mixture was immediately stirred and allowed to react in the dark at room temperature for 30 min. The absorbance of the reaction mixture was measured in a spectrophotometer at 734 nm. Trolox was used as a positive standard. The antioxidant activity was expressed in percentage of inhibition of the radical ABTS+.
DPPH assay: The antioxidant activity of the methanolic extract of C. filiformis using the DPPH radical (2,2′-diphenyl-1-picrylhydrazyl) was determined according to the method developed by Mensor et al. (2001). Accordingly, 0.8 mL of the methanolic solution of DPPH (0.118 mg/mL) was mixed with 2 mL of the seaweed extract (standard solution) in a test tube. The mixture was then immediately stirred and kept at rest for 30 min in the dark. The reaction mixture absorbance was measured in a spectrophotometer at 517 nm. Gallic acid was used as a positive standard. The antioxidant activity was expressed in percentage of inhibition of the DPPH radical.
The following equation was used in both cases:
A C = control absorbance
A M = absorbance of the reaction of the sample or the standard solution
A BM = absorbance of the blank sample or a blank of the standard solution
The EC50 value (mg/mL) is the effective concentration in which 50% of free radicals are neutralized. This value was calculated using a linear regression, whose value is the average of three measurements.
Statistical Analysis.- The experiments were performed in triplicate, and the results presented corresponded to the average ± SD. A regression analysis was used to calculate the EC50 value. Statistical comparisons were made using the analysis of variance using Paleontological Statistics program (PAST) version 2.17. The differences were considered significant when the p-value was less than 0.05 (p < 0.05).
Results and Discussion
Photochemical screening.- The general phytochemical study of the C. filiformis composition indicated nine groups of chemical compounds for both samples (i.e. Sechura Bay and Paracas Bay) (Table 1). Among these compounds, carbohydrates, substances of a phenolic nature (e.g. flavonoids and tannins), lipids, steroids, triterpenes, and alkaloids highlighting lipids in FAS and steroids in the FBS for the Sechura samples were present. According to the studies performed in other Caulerpa species, phenolic compounds (i.e., tannins and flavonoids), carbohydrates, terpenoids, and steroids were the common chemical groups present in Caulerpa (Karthick et al. 2014; Azhagu Raj et al. 2015).
Table 1 Preliminary phytochemical screening of the extracts of the composite of Caulerpa filiformis from Sechura Bay and Paracas Bay. (−): Negative, (+): Mild positive, (++): Moderate positive, and (+++): Marked positive.
| Fraction | Chemical groups | Sechura Bay | Paracas Bay | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Carbohydrates | ++ | ++ | Flavonoids | + | + | Lipids | +++ | ++ | Tannins | ++ | ++ | Phenolic oxidrils | ++ | ++ |
| B | Anthraquinones | − | − | Steroids | +++ | ++ | Triterpenes | ++ | ++ | Cardenolides | − | − | |||
| C | Alkaloids | ++ | ++ | Cardenolides | − | − | Leucoanthocyanidins | − | − | ||||||
| D | Quaternary ammonium salts | − | − | Steroids | − | − | Triterpenes | − | − | Flavonoids | − | − |
The secondary metabolites produced by Caulerpa possess antiviral (Nicoletti et al. 1999), anti-inflammatory (Stirk et al. 2003), antimicrobial (Vairappan, 2004), antitumor (Cavas et al. 2006), and antioxidant (Nguyen et al. 2011) properties. However, the concentration of these compounds can vary according to habitat, collection time, exposure to light, and availability of nutrients. Therefore, the results presented herein could vary if C. filiformis were collected at another time of the year.
Total phenol content.- Table 2 presents the yield and content of the total phenols of the methanolic extract of C. filiformis. The yield of the methanol extract of C. filiformis from Sechura Bay was lower than that obtained from Paracas Bay. However, the total phenolic content of the methanol extract of C. filiformis from Sechura Bay (39.31 ± 0.39 mg AGE/g of extract) was significantly higher (p < 0.05) than that obtained with C. filiformis from Paracas Bay (18.78 ± 0.31 mg AGE/g extract).
Table 2 Yield (% (w/w) of dry seaweed) and total phenol content (CFT) of the methanolic extract of Caulerpa filiformis from Sechura Bay and Paracas Bay. The CFT values are the mean ± standard deviation (SD), n = 3.
| Sampling site | Yield (%) | CFT (mg AGE/g extract) |
|---|---|---|
| Sechura Bay | 8.32 | 39.31 ± 0.39 |
| Paracas Bay | 15.72 | 18.78 ± 0.31 |
The results indicated that C. filiformis from Sechura Bay produced more than twice the amount of phenolic compounds compared to that from Paracas Bay, with samples obtained at the same time of the year. Similar values of the total phenol content in the range of 23.12 to 38.93 mg AGE/g seaweed extract were found in other Caulerpa species, such as C. racemosa, C. peltate, and C. taxifolia in India (Vinayak et al. 2011).
The phenolic compounds with a greater antioxidant activity in plants are flavonoids. These compounds have also been found in some algae. The antioxidant activity is not exclusive to phenolic compounds; other groups of chemical compounds, such as lipids and polysaccharides, also have antioxidant properties (Michalak and Chojnacka, 2015). Recent studies indicated that most marine algae produce chemical compounds with antioxidant activities (Chew et al. 2008), including some Caulerpa species, such as C. lentilifera and C. racemosa (Nguyen et al. 2011; Chia et al. 2015), whose activity is mainly attributed to phenolic compounds.
Antioxidant activity
ABTS radical uptake activity.- The antioxidant activity of the methanolic extract of C. filiformis from Paracas Bay was 79.60 ± 0.40%, whereas that from Sechura Bay was 78.49 ± 0.32%. By contrast, the antioxidant capacity in terms of IC50 (mg/mL) of the methanol extract from Sechura Bay was significantly higher (p < 0.05) compared to that from Paracas Bay, obtaining values of 2.55 ± 0.01 and 4.62 ± 0.01, respectively (Table 3). However, the antioxidant capacity of C. filiformis from both sites was significantly lower (p < 0.05) compared to the Trolox standard. Therefore, these results indicated that extracts of C. filiformis from Sechura Bay have a higher antioxidant capacity compared to those from Paracas Bay, although the latter presented a higher extract yield.
Table 3 Antioxidant activity of the methanolic extract of Caulerpa filiformis from Sechura Bay and Paracas Bay determined by the ABTS assay. The values are the average ± standard deviation (SD); n = 3; and AA = antioxidant activity.
| Sample | ABTS assay | ||
|---|---|---|---|
| AA ± DS (%) | EC50 (mg/mL) | ||
| Trolox standard | -* | 0.070 ± 0.001 | |
| C. filiformis from Sechura Bay | 78.490 ± 0.322 | 2.546 ± 0.007 | |
| C. filiformis from Paracas Bay | 79.599 ± 0.399 | 4.624 ± 0.014 | |
*=The test was not performed.
The antioxidant capacity of Caulerpa was studied in the form of extracts with an antioxidant potential, which varied in the same or different species (e.g., C. racemosa exhibited the capacity of trapping free radicals (ABTS) with an IC50 = 0.709 ± 0.02 mg/mL, greater than that obtained in the analyzed samples). However, similar studies in other algae (i.e. P. australis and S. polycystum) indicated that brown algae have a greater antioxidant potential compared to C. racemosa (Gany et al. 2014).
DPPH radical uptake activity.- The antioxidant activity of the methanolic extract of C. filiformis from Paracas Bay was 84.65 ± 1.56%, whereas that from Sechura Bay was 72.53 ± 1.50%. By contrast, the antioxidant capacity in terms of IC50 (mg/mL) of the methanol extract from Sechura Bay was higher compared to that from Paracas Bay, with values of 2.19 ± 0.02 and 3.22 ± 0.05, respectively (Table 4). However, the results of the antioxidant activity obtained from both sites of C. filiformis were significantly lower (p < 0.05) compared to the standard gallic acid.
Table 4 Antioxidant activity of the methanolic extract of Caulerpa filiformis from Sechura Bay and Paracas Bay determined by the DPPH assay. The values are the average ± standard deviation (SD); n = 3; and AA = antioxidant activity.
| Sample | DPPH assay | |
|---|---|---|
| AA ± DS (%) | EC50 (mg/mL) | |
| Gallic acid standard | - | 0.009 ± 0.001 |
| C. filiformis de Sechura | 72.014 ± 0.601 | 2.194 ± 0.021 |
| C. filiformis de Paracas | 84.651 ± 2.631 | 3.218 ± 0.054 |
In this assay and in the ABTS assay, the extract of C. filiformis from Sechura Bay evidently had a greater antioxidant capacity compared to that from Paracas Bay. Similar studies in Caulerpa also showed a greater ability of the extract to trap free radicals (DPPH).
Correlation between antioxidant activity and phenol content.- The antioxidant activity of the macroalgae extracts could be mainly related to the content of phenolic compounds without ruling out the synergistic action between their compounds (Chew et al. 2008; Abdallah et al. 2017). Therefore, the correlation between the antioxidant activity of C. filiformis and the phenol content was determined, finding a direct and significant correlation for both the ABTS test (ρ (rho) = 0.641, p = 0.025) and DPPH (ρ) (rho) = 0.625, p = 0.03).
Conclusions
In the present study, C. filiformis from Sechura Bay and Paracas Bay was proven to be an important source of chemical groups, mainly phenolic compounds. A higher extract yield was obtained in C. filiformis from Paracas Bay. The total phenolic content of the methanol extract of C. filiformis from Sechura Bay was significantly higher compared to that from Paracas Bay. In the ABTS and DPPH assays, the methanolic extract of C. filiformis from Paracas Bay had a significantly higher percentage of inhibition of radicals (p < 0.05) compared to that from Sechura Bay. However, the antioxidant capacity (EC50) of the Sechura extract was significantly higher (p < 0.05) than that of Paracas Bay, indicating that the extract of C. filiformis from Sechura Bay has a greater antioxidant capacity.












 uBio
uBio