Introduction
The industrial uses of microbial enzymes (e.g., food, agriculture, chemicals, and pharmaceuticals) has gained interest and increased rapidly in comparison to plant and animal enzymes (Singh et al. 2016). In this context, waste could be an important source of bacteria and enzymes diversity with potential biotechnological applications.
Bacteria and their enzymes can also be used as an alternative to chemical methods in different industrial processes being an environmentally friendly technology (Singh et al. 2016). Some microbial enzymes with special characteristics of industrial importance include proteases, keratinases, xylanases, amylases, lipases and chitinases (de Souza & de Oliveira e Magalhães 2010, Rathore et al. 2015). Isolation of microorganisms of new sources would provide enzymes possessing special characteristics for use in various bio-industries requiring these enzymes (Nigam 2013).
Bacteria and microbial enzymes could also be useful to solve environmental problems caused by industrial activities (Singh et al. 2016). One of the industries in need of a strategy to reduce the environmental impact produced by the generated waste is the shrimp aquaculture industry (Páez-Osuna 2001). The use of specific microbial enzymes to address this problem could be an interesting environmentally friendly approach.
Shrimp aquaculture production provides an acceptable protein rich supplement for human consumption (Kandra et al. 2012). However, a large amount of shrimp shell-waste is also generated by shrimp farms during this activity (Dai et al. 2015). Shrimp waste is composed mainly by proteins (40%), lipids (10%), minerals (28%) and chitin (17%) (Synowiecki & Al-Khateeb 2000). Carapace (head shells) and abdominal (tail) shells constitute the shrimp shell-waste (Uno et al. 2012). This shrimp shell-waste is removed in peeling sheds near the landing or at packaging plants (Kandra et al. 2012). The cephalothorax and the exoskeleton constitute 60% of the animal (ITP & IMARPE 1996), depending on species. Countries dedicated to carniciculture generate approximately up to 48 56% of the raw shrimp weight depending on species (Singla and Chawla 2001, Pu et al. 2010), which if not dealt with, generates a problem of environmental contamination.
Several studies have been described for chitinolytic bacteria with the ability to degrade shrimp-shell waste (Setia 2015) and lactic acid fermentation processes for shrimp head waste to be used as silage meal and for chitin recovery (Kandra et al. 2012). Isolation of bacterial strains that have specific enzymatic capacities from shrimp shell waste would have the advantage to accelerate the degradation process of this substrate to obtain products for future applications such as the recovery of chitin, pigments, essential amino acids, and fatty acids, to be used in medical, cosmetics, paper, pulp, textile, and food industries (Kandra et al. 2012). The aim of the present study was to isolate and identify bacterial strains with proteolytic, amylolytic, lipolytic and chitinolytic capacities from shrimp waste.
Material and methods
Sample collection and processing
Shrimp (Lito-penaeus vannamei) waste from a company dedicated to produce and commercialize hydrobiological products, located in Tumbes Perú, was used in the present study as a source of bacterial strains. This waste, composed of cephalotorax and abdominal exoskeletons, was collected (July, 2015) and placed into seawater broth under sterile conditions. Samples were sent in a cooler box to the Laboratory of Biotechnology in ITP, where they were immediately processed.
Bacterial isolation and purification
Shrimps cephalothorax (50 g) were transferred to a bottle containing 150 mL of seawater broth (SWB, 10 g meat extract, 10 g peptone, 750 mL filtered seawater and 250 mL distilled water, pH 7.5 8.0) and mixed in a sterile homogenizer. The mix was incubated at 25 °C for 72 h. Dilutions were made from the sample bottle using the method described by Carvajal et al. 1991. Five serial decimal dilutions were made (10-1 to 10-5) in test tubes containing 9 mL of SWB. An aliquot of 0.1 mL of each dilution was spread onto seawater agar (SWA) plates. The plates were incubated at 25 °C for 24 h. Colonies with different morphological characteristics were selected. Isolated bacterial strains were characterized by standard microbiological procedures: Gram staining, colonial morphology, catalase test and cytochrome oxidase reaction.
Bacterial screening for enzymatic capacity
Screening for extracellular enzymatic capacity (amylase, chitinase, lipase, and protease) was conducted on agar plates supplemented with specific enzyme substrates, such as starch, chitin, tributyrin, gelatin and casein. A transparent halo surrounding colonies would indicate enzymatic capacity. Tests were performed by duplicate.
Hydrolysis of starch
To determine strains with amylolytic capacity, the method of Skerman (1969) was used. An aliquot of 5 μL of bacterial culture (18 24 h) was spread onto SWA plates supplemented with 0.2% soluble starch (w/v) and incubated at 25 °C for 5 days. Colonies were then covered with a lugol solution.
Hydrolysis of tributyrin
Lipolytic capacity of the strains was determined by the method of Cardenas et al. (2001). An aliquot of 5 μL of bacterial culture (18 24 h) was spread onto tributyrin agar plates containing 1% trybutirin and supplemented with 3% NaCl (w/v); then incubated at 25 °C for 5 days.
Hydrolysis of casein
To determine proteolytic capacity, the method described by Ryden et al. (1973) was used. An aliquot of 5 μL of bacterial culture (18 24 h) was spread onto milk agar plates (3% skim milk powder) and incubated at 25 °C for 5 days.
Hydrolysis of gelatin
Proteolytic capacity was determined by the method of Frazier (1926). An aliquot of 5 μL of bacterial culture (18 24 h) was spread onto SWA plates supplemented with 1.5% microbiological gelatin and incubated at 25 °C for 5 days. A mercuric chloride solution (HgCl ) acidified with concentrated HCl, was added onto the agar plates until colonies were completely covered.
Hydrolysis of chitin
Chitinolytic capacity was determined by the method of Wirth and Wolf (1990). An aliquot of 5 μL of bacterial culture (18 24 h) was spread onto chitin agar plates (2% chitin) and incubated at 25 °C for 5 days.
Dendrogram
A dendrogram of cluster analysis on the degradation of substrates by bacterial strains was produced by the DendroUPGMA tool (a dendrogramconstruction utility, http://genomes.urv.cat/UPGMA/) using the Euclidean coefficient and UPGMA method.
Biochemical and molecular identification of bacterial isolates
Biochemical tests were conducted to identify bacterial strains, by using the API 20 NE identification system according to the protocol supplied by the manufacturer. Strains with the best enzymatic capacities for each substrate were molecularly identified for future uses in industrial processes. Total DNA from those selected strains was extracted by using the PureLink Genomic DNA Mini Kit (Invitrogen). Partial 16S rDNA was sequenced by Macrogen (USA). DNA amplification by the polymerase chain reaction (PCR) method was performed using primer 27 F (5’-AGAGTTTGATCCTGGCTCAG-3’) and primer 1492 R (5’-GGTTACCTTGTTACGACTT-3’) according the protocol of Reysenbach, Longnecker and Kirshtein (2000). The resulting sequences were compared with those from the GenBank database in order to determine similarities by using the BLASTN algorithm. Sequences of 16S rDNA obtained in the present work were submitted to the GenBank database: Bacillus velezensis RL15-42 (MK993377); Shewanella sp. RL15-19 (MK993378); Shewanella sp. RL15-10 (MK993379); Shewanella sp. RL15-41 (MK993380); Vibrio parahaemolyticus RL15-44 (MK993381); Vibrio parahaemolyticus RL15-22 (MK993382), and Vibrio parahaemolyticus RL15-50 (MK993383).
Multiple alignment of 16S rDNA sequences of selected strains from the present work and reference bacterial sequences from the GenBank database (https://www.ncbi. nlm.nih.gov/genbank/), was performed using ClustalX2 (Larkin et al. 2007). Phylogeny trees were constructed using the Neighbor-Joining method, Kimura 2-parameter model with 1,000 bootstrap replicates on the MEGA v5 program (Tamura et al. 2011). Bacteria of different genera were used as outgroup.
Comparison between extracellular enzymatic capacity of strains isolated from shrimp waste and the predicted enzymatic capacity of strains from the GenBank database
Amino acid sequences of proteolytic, lipolytic, amylolytic and chitinolytic enzymes were selected from the GenBank database genomes of the same species identified in the present study. Those genomes were selected due to their high number of protein annotated in relation to other strains. Those enzymes amino acid sequences were scan by sub-cellular localization using PSORTb 3.0 (Yu et al. 2010), CELLO v.2.5 (Yu et al. 2006) and Gram-LocEN (Wan et al. 2017) programs to determine extracellular localization. Results were expressed as capacity to produce extracellular enzymes found in the database genomes and compared to the capacities of strains isolated in the present study.
Results
Isolation and purification of bacterial strains
Thirty two bacterial strains were isolated from shrimp waste, 31 Gram negative and 1 Gram positive. All 32 strains were positive for catalase and 31 were positive for cytochrome oxidase tests (Table 1).
Enzymatic capacity
Bacteria isolated from shrimp waste, showed different profiles for lipase, protease, amylase and chitinase capacities on agar plates (Figure 1 A and Table 1). Nine bacterial strains showed amylolytic capacity on starch agar plates. Halos diameter ranged from 12 to 35 mm; 44.44% (4) of the strains showed halos diameter ≥ to 24 mm. Strains with the best amylolytic capacity were RL15-22 and RL15-42 (Table 1).
Twenty eight out of 32 bacterial strains presented lipolytic capacity on tributyrin agar plates. Halos diameter ranged from 7 to 13 mm; 25% (7) of the strains showed halos diameter ≥ to 12 mm. Strains with the best lipolytic capacity were identified as Bacillus velezensis (RL15-42) and Vibrio parahaemolyticus (RL15-50, RL15-54 and RL15-55) (Table 1).
Twenty-nine bacterial strains showed proteolytic capacity on casein and 31 on gelatin agar plates. Halos diameter ranged from 10 to 32 mm and from 20 to 46 mm, respectively; 55.17% (16) of the strains showed halos diameter ≥ to 23 mm on milk agar plates and 58.06% (18) were ≥ to 27 mm on gelatin agar. Strains with the best proteolytic capacity were RL15-44 and RL15-10 on gelatin and RL15-41 and RL15-19 on casein (Table 1).
Nine bacterial strains showed chitinolytic capacity after 5 days of incubation. Halos diameter ranged from 11 to 14 mm. The two strains with the best chitinolytic capacity were RL15-50 and RL15-44 (Table 1). Strains with the best activities for each substrate were chosen to be molecularly identified by 16S rDNA sequencing.
In general, 96.88% of studied bacterial strains (6 genera) showed proteolytic capacity, 87.50% lipolytic, 28.13% amylolytic and 28.13% chitinolytic capacity (Fig. 1B).
Dendrogram of cluster analysis on isolated strains enzymatic capacities
Figure 2 is a dendrogram showing clusters of the isolated bacterial strains based on enzymatic profile similarities to degrade specific substrates. The strains were distributed into two main clusters, one with the strain Burkholderia cepacia that showed lipolytic capacity only, and the other cluster with the genera Shewanella, Ochrobactrum, Pseudomonas, Vibrio, Bacillus and 2 strains of B. cepacia, this last one, showed enzymatic capacity to degrade 2 and 3 substrates.
Each one of the bacterial strains studied in the present work, showed enzymatic capacity for 1, 2, 3 or all 4 of the tested enzymes (Table 1, Fig. 2). Lipolytic and proteolytic capacities were found in all studied strains, except B. cepacia (RL15-46) and some Shewanella sp. (RL15-04, RL15-09, RL15-16 and RL15-07). In contrast, amylolytic capacity was found in Vibrio species and Bacillus velezensis only. Chitinolytic capacity was shown by one Shewanella sp., B. velezensis and Vibrio spp. (Fig. 2).
Table 1 Enzymatic capacity of bacterial strains isolated from shrimp waste
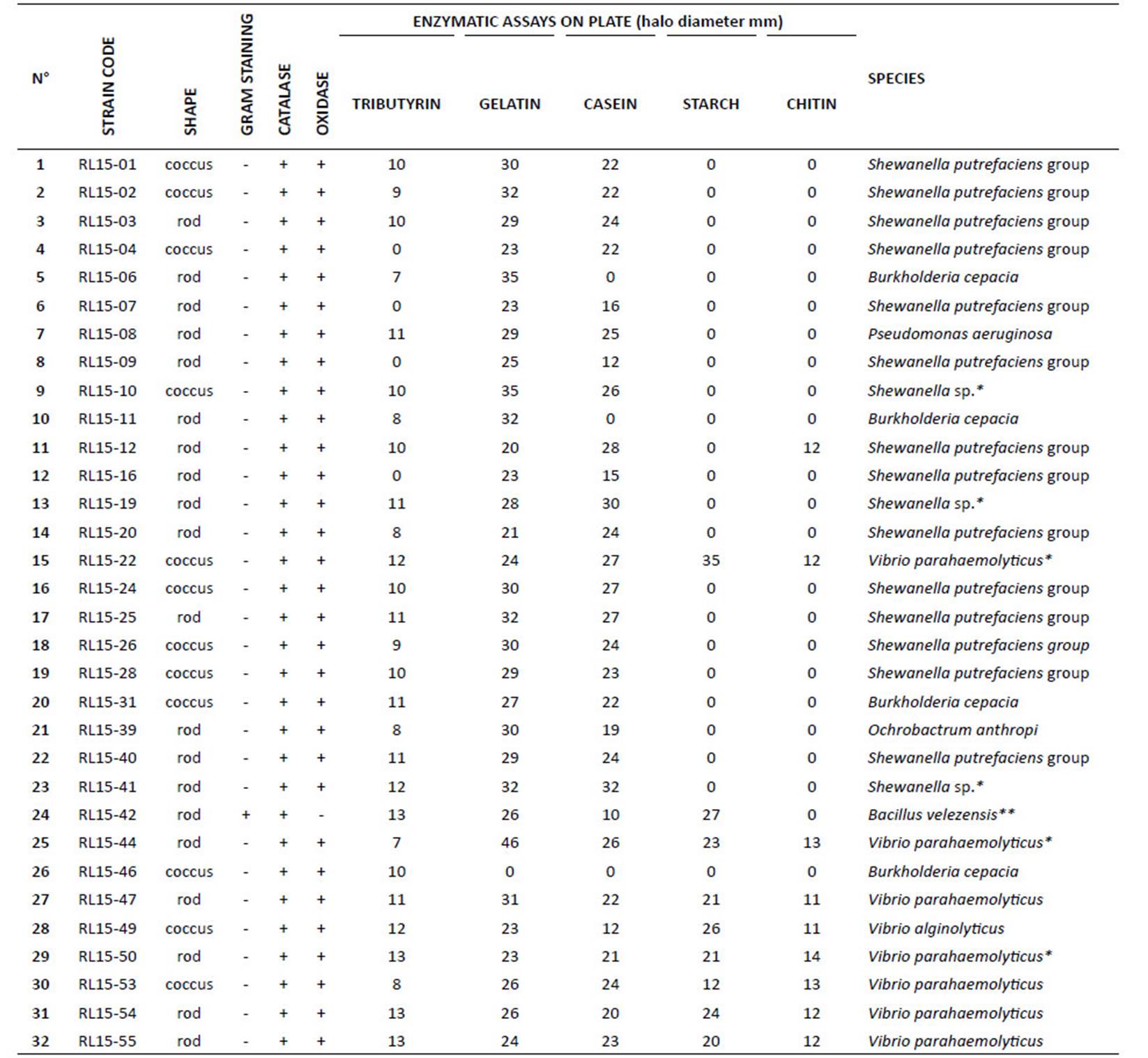
(*) Strains identified by 16S rDNA sequencing and the API system. (**) Strain only identified by 16S rDNA sequencing. All other strains were identified by the API system.
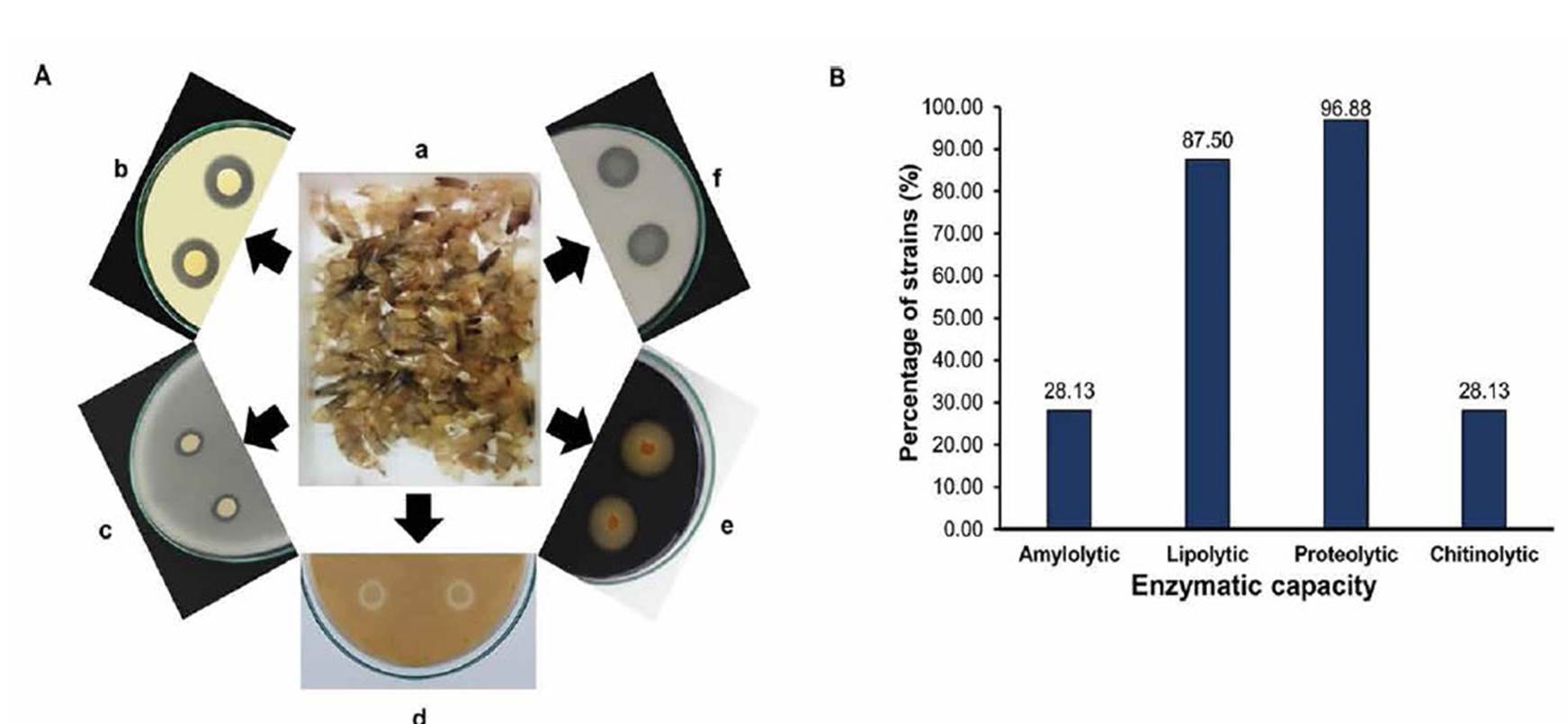
Figure 1 Lipase, protease, amylase and chitinase plate assays on bacteria isolated from shrimp waste and enzymatic percentage distribution. A: (a) Shrimp waste, (b) RL15-31: Halo of transparency on Gelatine agar, (c) RL15-49: Halo on Tributyrin agar, (d) RL15-55: Halo on Chitin agar, (e) RL15-44: Halo on Starch agar, (f) RL15-41: Halo on Casein agar. B: Percentage distribution of enzymes produced by bacterial strains isolated from shrimp waste.
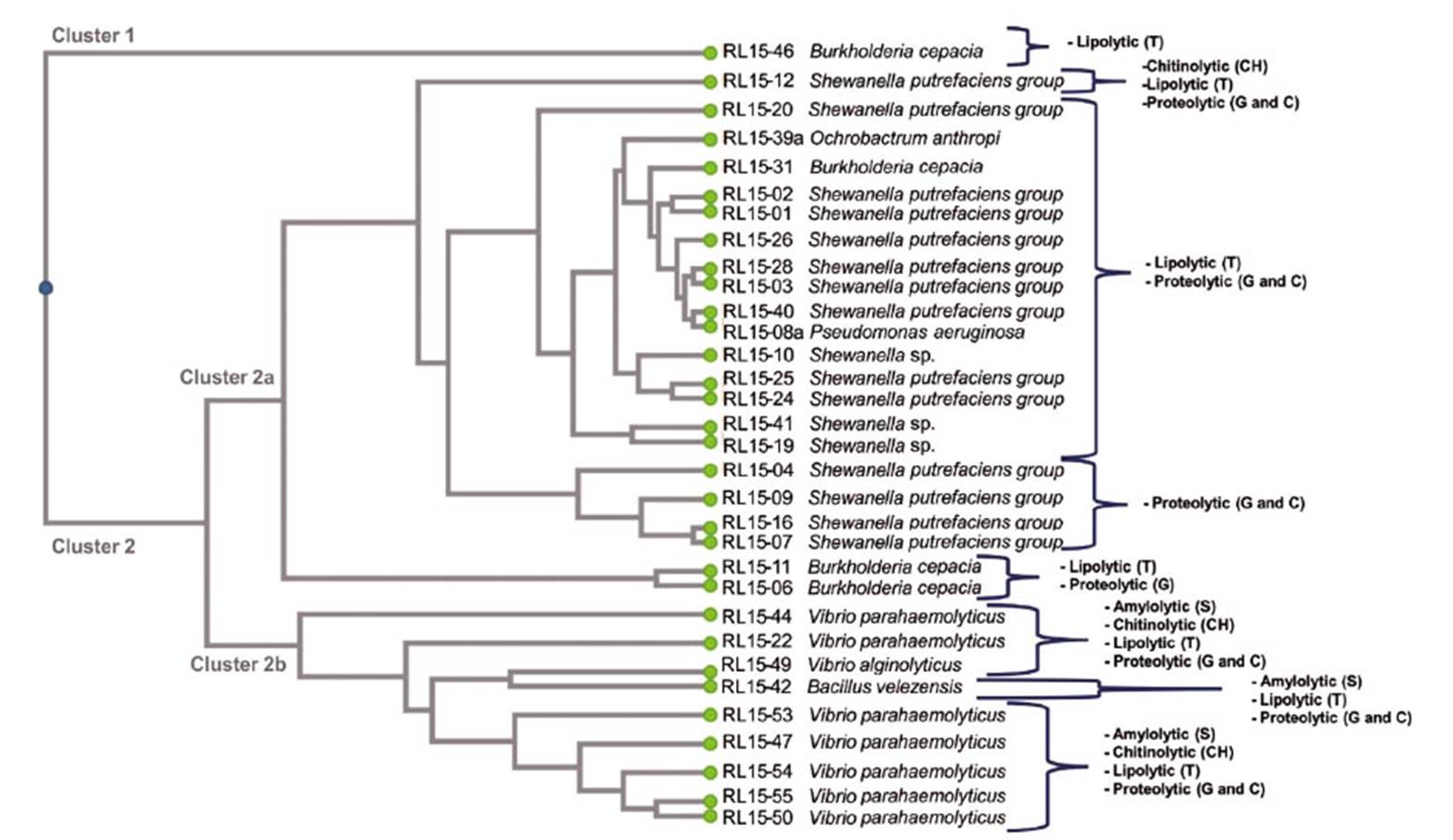
Figure 2 Dendrogram based on degradation of substrates (G: Gelatin, C: Casein, T: Tributyrin, S: Starch and CH: Chitin) by strains isolated from shrimp waste. Enzymatic profiles are indicated. Euclidean coefficient and UPGMA method were used from the DendroUPGMA tool (a dendrogramconstruction utility).
Identification of bacterial species
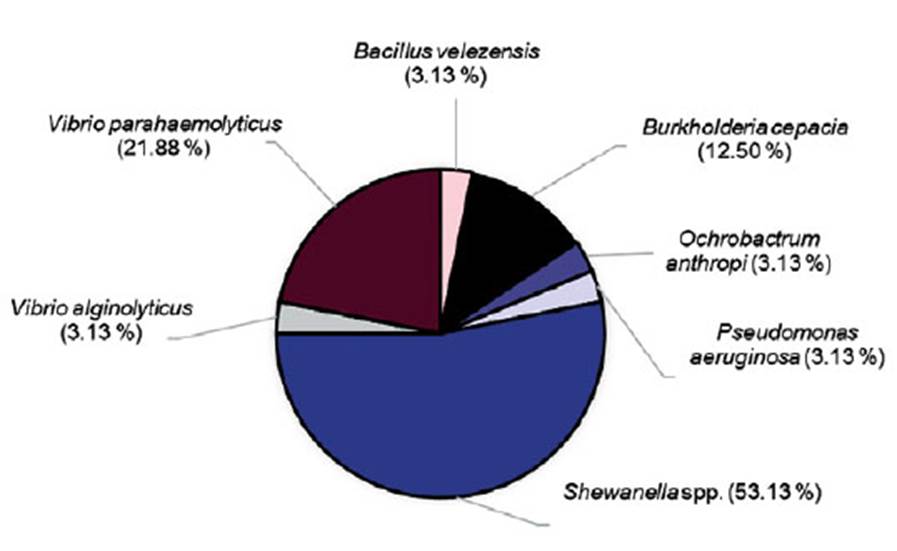
Out of the 32 bacterial strains isolated from shrimp waste, 53.13% (17 strains) were identified as Shewanella spp., 21.88% (7 strains) as Vibrio parahaemolyticus, 12.50% (4 strains) as B. cepacia, and 12.52% (4 strains) as each one of the following species: B. velezensis, Vibrio alginolyticus, Ochrobactrum anthropi, and Pseudomonas aeruginosa (Figure 3).
Identification of bacterial strains with the best enzymatic capacity, as well as similarity by BLASTN and phylogenetic analysis based on 16S rDNA gene sequences, revealed that strains RL15-41, RL15-10 and RL15-19 belonged to the genus Shewanella and were closely related to Shewanella algae, Shewanella upenei and Shewanella haliotis with 99.15 100% identity percentage for these species (Fig. 4). Thus, these isolated strains were designated as Shewanella spp. Strains RL15-50, RL15-22 and RL15-44 were identified as V. parahaemolyticus with 99.7, 99.93 and 100% identity, respectively. Strain RL15-42 was identified as B. velenzensis with 99.02% identity, which is a species synonym of B. methylotrophicus (Dunlap et al. 2016).
Comparison of extracellular enzymatic capacity between isolated bacterial strains and selected strains from the GenBank database
Different extracellular enzymes were identified among bacterial strains from the GenBank database and from those isolated in the present study (Table 2). Proteolytic enzymes genes were present in all analyzed genomes from the GenBank database, except Ochrobactrum anthropi. Lipolytic capacity was identified in B. cepacea, P. aeruginosa, B. velezensis and V. parahaemolyticus genomes. Amylolytic capacity was identified in B. velezensis and V. parahaemolyticus genomes. Chitinolytic capacity was identified in P. aeruginosa and V. parahaemolyticus genomes. Enzymatic profiles of strains isolated in the present study were different from those from the GenBank database (Table 2).
Discussion
Bacteria isolation from the same source in need of treatment itself, would allow to obtain strains with specific characteristics already adapted to that particular source, with the advantage of performing better than strains obtained from any other source, for a specific industrial purpose. In the present study, bacteria were isolated from shrimp waste, to be used in future shrimp waste treatment. Among them were B. cepacia, O. anthropi, P. aeruginosa, V. alginolyticus, V. parahaemolyticus, B. velezensis and Shewanella spp. Shewanella genus have been described among the predominant spoilage bacteria in refrigerated shrimp (Litopenaeus vannamei) (Zhu et al. 2018). Burkholderia cepacia has also been isolated from marine environments (Alkhunni et al. 2017). Strains of Vibrio are ubiquitous in marine and estuarine aquatic ecosystems in which shrimp occur naturally and are farmed (Gopal et al. 2005), therefore, these bacteria are considered opportunistic pathogens of shrimp (Kraxberger-Beatty et al. 1990; Woo et al. 2015). Species of the genus Bacillus have also been isolated from shrimp waste such as Bacillus cereus and Bacillus amyloliquefaciens (Sorokulova et al. 2009; Setia 2015), as well as B. velezensis, isolated from the same type of source in the present work.
Isolated bacterial strains from shrimp waste showed amylolytic, lipolytic, proteolytic and chitinolytic capacities. Hydrolytic profile of microorganisms depends of the source where they have been isolated from and the composition of the substrate for the enzyme of interest (Vishwanatha et al. 2010, Muthulakshmi et al. 2011). Different substrates such as effluents of dairy and meat processing industry (Sangeetha et al. 2012, Mazzucotelli et al. 2013), soil contaminated with oil, oilseeds, and tannery effluent (Siva & Abraham 2011), have been used to obtain bacteria with hydrolytic capacity. Our results suggest that shrimp waste is a rich substrate to obtain a good diversity of bacterial enzymes (amylolytic, lipolytic, proteolytic and chitinolytic enzymes), interesting for the biotechnology industry.
Amylolytic bacteria studied in the present work, showed to be mostly Vibrio species (V. parahaemolyticus and V. alginolyticus), but also B. velezensis. These results agree with those reported by other authors for amylolytic capacity from different Vibrio species (Raghul Subin & Bhat 2011). Like our results, the genus Bacillus has been reported with amylolytic capacity (Zhou et al. 2018). In addition, several bacteria including B. cepacia, O. anthropi, P. aeruginosa, B. velezensis, Shewanella spp., V. alginolyticus and V. parahaemolyticus showed the ability to produce lipolytic enzymes. Our results are in accordance with the report of a wide variety of species (sub)phyla including Betaproteobacteria (including Burkholderia) and Gammaproteobacteria (including Pseudomonas and Vibrio), which are known to be some of the main microbial sources of commercially available lipolytic enzymes (Hassan, Abdul-Raouf and Abdel-Rahiem Ali 2015; Narihiro et al. 2014).
Proteolytic and chitinolytic bacteria were also isolated in the present study. Brzezinska et al. (2008) also reported bacteria that produce proteases and chitinases from shrimp by-products. In our study, Shewanella spp., V. alginolyticus, and V. parahaemolyticus showed the ability to produce chitinolytic enzymes. S. putrefaciens, Vibrio aestuarianus and Streptomyces sp. isolated from crustacean waste have also been reported as chitinolytic species (Sastoque-Cala et al. 2007, Anuradha & Revathi 2013).
Table 2 Enzymatic capacity comparison between strains isolated from shrimp waste and the predicted enzymatic capacity of strains from the GenBank database
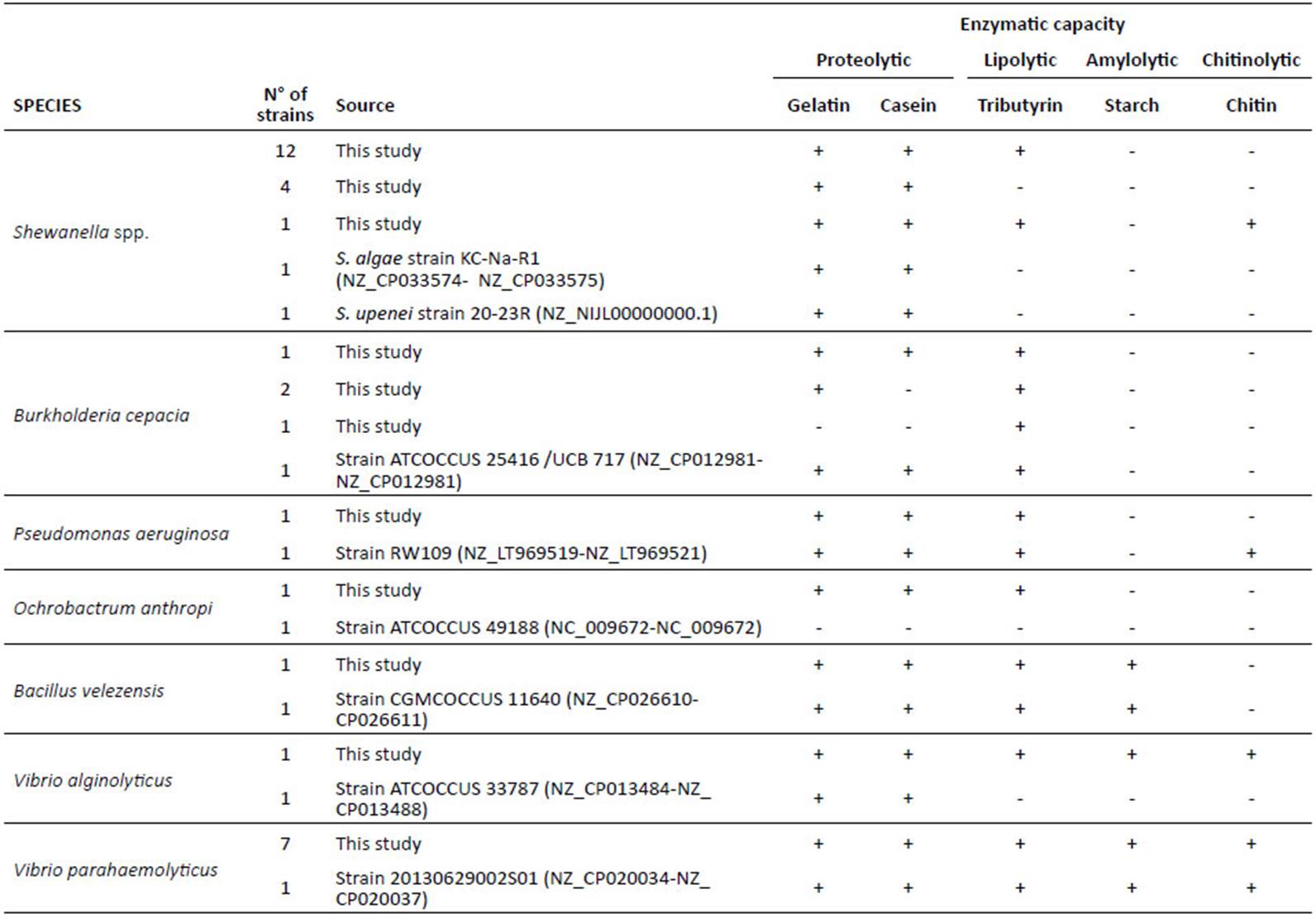
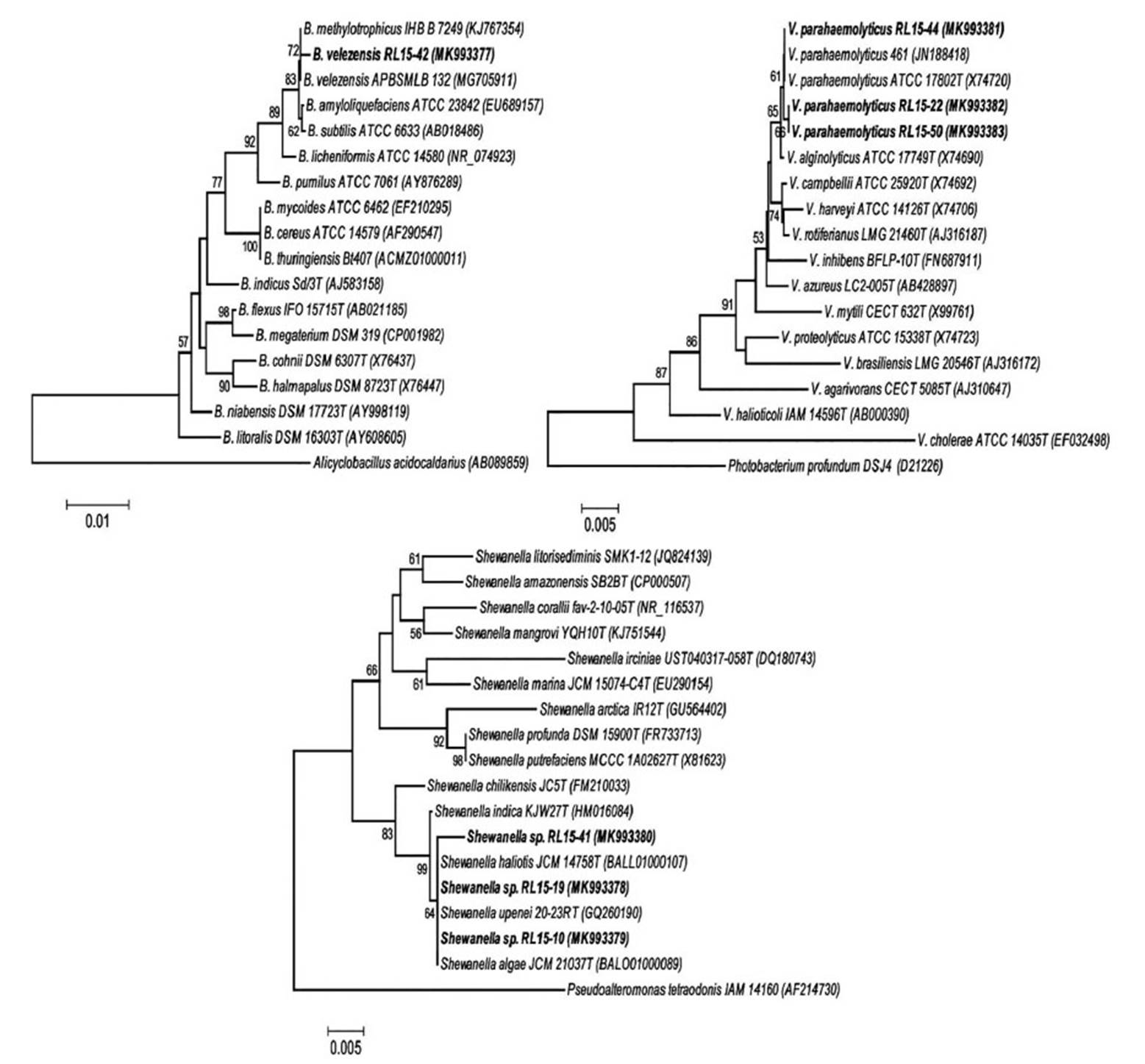
Figure 4 The tree was constructed using the Neighbor-Joining method and genetic distances were generated using the Kimura 2-parameter model. Values on the branches represent the percentage of 1000 bootstrap replicates. Bootstrap values over 50% are shown. Bacterial strains from shrimp waste are shown in bold. GenBank accession numbers are in parentheses. Alicyclobacillus acidocaldarius, Photobacterium profundum and Pseudoalteromonas tetraodonis were used as outgroup to root the trees.
Bacterial strains with proteolytic capacity found in the present study were Shewanella spp., including S. putrefaciens group, P. aeruginosa, V. alginolyticus, V. parahaemolyticus, B. cepacia, O. anthropi and B. velezensis. S. putrefaciens, involved in marine fish decomposition, have shown high extracellular protease activity suggesting the presence of several proteases (Odagami et al. 1994). In addition, species of Vibrio including V. alginolyticus and V. parahaemolyticus, are known to produce several extracellular proteolytic enzymes (Miyoshi 2013). In a previous study, strains of Vibrio and Shewanella producing proteases were isolated from marine sediment samples (Zhou et al. 2015). We describe strains with proteolyitic but no chitinolytic capacity among different genera of bacteria, including Bacillus, which could be used for waste deproteinization processes. For example, chitinase-deficient Bacillus licheniformis strains can efficiently deproteinizate shrimp shell waste, resulting in chitin of superior quality (Waldeck et al. 2006). It should be mentioned that the majority of studies on crustacean waste have been carried out to isolate only chitinolytic bacteria (Waldeck et al. 2006; Anuradha & Revathi 2013; Setia 2015).
Based on our results, bacterial strains with a diversity of extracellular enzymes were found in shrimp waste. For instance, some B. cepacea isolated strains were able to hydrolyze gelatin and/or casein. In addition, most of the enzymatic profiles of strains isolated in the present study were different from those of the same species predicted from the GenBank genomes database. In this context, we did not find chitinolytic enzymes for P. aeruginosa in comparison to the chitinolytic potential of P. aeruginosa RW109 from the database, identified through bioinformatic analysis of extracellular enzymes. However, extracellular enzymes found in B. velezensis strain CGMCC 11640 and V. parahaemolyticus strain 20130629002S01 by bioinformatics analysis, showed that these strains have the potential to hydrolyze similar substrates to those hydrolyzed by our strains. Our results show an enzymatic diversity among the bacterial community isolated from shrimp waste. It has been observed that strains isolated from different environments could have different enzymatic phenotypes, although they belong to the same species.
In summary, we identified 32 bacterial strains (represented in 6 genera) with different enzymatic profiles. The most representative enzymatic capacities among the isolated strains were proteolytic (96.88%) and lipolytic (87.50%). The genus Vibrio showed to produce all the enzymes tested (proteases, lipases, amylases and chitinases). V. parahaemolyticus strains showed the highest proteolytic, amylolytic, lipolytic and chitinolytic capacity. Strains isolated from a particular substrate (e.g., shrimp waste) would have specific enzymes to hydrolyze that substrate, therefore could be used as an alternative not only to chemical methods, but also to bacterial strains obtained from a different source. They could be used in industrial processes (e.g., deproteinization process of shrimp waste to obtain chitin). Moreover, genes coding for enzymes produced by these bacteria, could be cloned and expressed in a suitable cell host, the enzymes could be characterized and used with biotechnological purposes in order to improve industrial bioprocesses












 uBio
uBio