Introduction
Ecuador is considered a biodiversity hotspot, containing approximately 4718 vertebrate and seven main types of ecosystems (Tirira 2017). Unfortunately, Western Ecuador has suffered several environmental problems over a prolonged period, such as deforestation, illegal hunting, and rapid change in land use (Dodson & Gentry 1991, Parker & Carr 1992), causing declines in mammalian species. The loss of native mammals has pervasive cascading effects on ecosystems (Redford 1992, Dirzo et al. 2014). For example, the loss of large herbivores affects seed dispersal and recruitment, the loss of carnivores decreases predatory activity and nutrient recycling, and the decline of other mammalian species impacts pollination (Rumiz 2010). Thus, to aid in mammal management and conservation, it is important to document current species richness and evaluate potential threats (Cervera et al. 2016, Lizcano et al. 2016).
Mammals in Ecuador have been best documented in the Amazon region (Albuja et al. 2012, Brito et al. 2019); provinces such as Napo and Morona Santiago show a high mammal diversity of 206 species (Brito et al. 2019). Around 140 species have been reported in Western Ecuador, including recent reports and updates in several protected areas, particularly in dry forest (Espinosa et al. 2016, Cervera et al. 2016, Lizcano et al. 2016, García-Olaechea et al. 2021). These inventories highlight the importance of protected areas that serve as refuges for populations in the latter region (Solórzano et al. 2021).
Western Ecuador has also been identified as an area of primary concern since it contains irreplaceable forest remnants that are crucial for conservation efforts (Mittermeier et al. 2004, Solórzano et al. 2021). Consequently, there are 21 protected areas that include coastal-marine landscapes, mangroves, the humid forest of Chocó, and Tumbesian dry forests (MAE 2015). One of these areas is Isla Santay, created and managed since 2010 by the Ministry of Environment, Water, and Ecological Transition of Ecuador (known by its acronym in Spanish, MAATE) to preserve endemic and threatened species as well as habitats such as wetlands, scrub, and mangroves; the area is also historically and culturally significant for the Guayas province (MAE 2011). Before the COVID-19 pandemic, it was one of the most-visited tourist sites in Ecuador, receiving 4000 monthly visits (SIB 2018).
Previous knowledge regarding mammals in the Isla Santay protected area is very scarce, unsystematic, anecdotal, and outdated (Jaramillo et al. 2002, MAE 2011). This information gap must be filled to better understand the conservation status of mammals in this protected area and the region. Herein, we identify and analyse the richness and abundance of large and medium-sized mammals on Isla Santay in two zones with different levels of disturbance; subsequently, we discuss threats and conservation priorities.
Material and methods
Study site. - Isla Santay and Isla Gallo National of Recreation Area (known by its abbreviated form, Isla Santay) is part of the National System of Protected Areas of Ecuador (known by its acronym in Spanish, SNAP), and it is also a Ramsar Wetland of International Importance (MAE 2011). Isla Santay is located at the head of the Guayas River (2°13’ S; 79°51’ W), between three large cities in the province of Guayas: Samborondón, Durán and Guayaquil (Fig. 1).
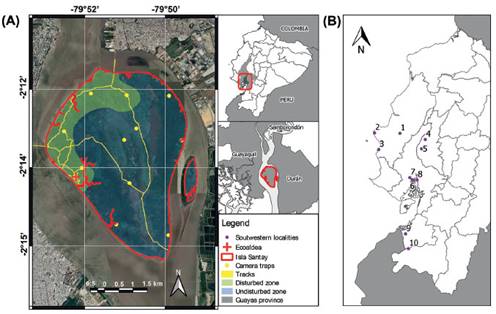
Figure 1 (A) Map of the Isla Santay and Isla Gallo National Recreational Area. The legend indicates the location of the camera-trap stations, transects, and study zones from August 2018 to January 2019. (B) Other localities in Southwestern Ecuador are included: 1) Refugio Valle Alto, 2) Refugio de Vida Silvestre Marino - Costera Pacoche, 3) Parque Nacional Machalilla (Manabí province), 4) Bosque Protector Pedro Franco Dávila o Jauneche, 5) Humedal Ramsar Abras de Mantequilla (Los Ríos province), 6) Reserva de Producción de Fauna Manglares El Salado, 7) Bosque Protector Cerro Blanco, 8) Área Nacional de Recreación Isla Santay y del Gallo (Guayas province), 9) Reserva Ecológica Arenillas, 10) Bosque Protector Petrificado Puyango (El Oro province).
The island has an altitude range of 0 to 10 m, with an area of approximately 2214 ha (MAE 2011). The main landscapes present on the island are mangroves, flooded zones, and estuarine tidal flats; remnants of scrub and dry forests are predominant in the inland region (Jaramillo et al. 2002). The climate is warm year-round, and the temperature oscillates between 20 and 27 °C; the island experiences two annual seasons: rainy (from December to May) and dry (from June to November) (Jaramillo et al. 2002). In the past, the island inhabitants cultivated rice crops and livestock; currently, the descendants of these farmers live in the San Jacinto community, or “Eco-Aldea,” and their main industrial activities include fishing and ecotourism.
Data collection. - The sampling area was divided into two zones: (1) disturbed, encompassing the Eco-Aldea and surroundings and utilized for tourism activities; and (2) undisturbed, where access is granted to rangers and researchers but not to visitors or tourists (Fig. 1 and 2). We conducted direct and indirect samplings from August 2018 to October 2018 and recorded data via camera traps from November 2018 to January 2019 (Research Authorization 029-2018-IC-FLO/FAU-DPAG/MAE, granted by Ministerio del Ambiente de Ecuador).
For direct sampling, we established two line transects per zone; each transect was approximately 6 km long and 4 m wide. We conducted six surveys of these transects: three during the daytime (8:00 - 14:00) and three at night (19:00 - 23:00). For indirect sampling, we searched for signs of mammals, such as footprints, scats, burrows, scrapes and scratches, or skeletal remains (Tirira 2017).
Camera-trapping. - Using QGIS version 2.18 (QGIS 2016), we established 11 sampling stations, dividing up the protected area using a 1.5 km grid (Fig. 1) as recommended by Lira-Torres and Briones (2012). We used nine Bushnell® Trophy Cam trail cameras and two Covert DLC 2915 trail cameras. The cameras were placed in a north-south direction to avoid solar interference (Swan et al. 2014). We set each camera trap to be active 24 hours a day, with a three-second interval between each detection to maximize the number of detections. We placed the cameras 30 cm above ground level and left them in the field for 92 days. To minimize our presence at the sampling points, we checked the cameras every 15 days (Zamora 2012). To ensure the independence of detections, we set a 1-hour interval between each photograph. The mammal species identification was based on Tirira (2017) and the taxonomic arrangement follows Tirira et al. (2021).

Figure 2 Camera-trapping stations. Undisturbed zone: A) scrub and herbaceous, C) inner wetlands, D) dry forest remnants, E) Embankment o “terraplén”; Disturbed zone: B) Visitor trail. All photos by ATD.
Data analyses. - We estimated the Relative Abundance Index (RAI) by compiling all indirect and direct records (including camera trapping records) using the following formula (Carrillo et al. 2000):
Relative Abundance Index = Number of records /unit of effort
Number of records corresponds to the number of tracks, scats, skeletal remains, sightings, and scratches (direct and indirect sampling) and the number of camera trap records.
Unit of effort corresponds to the meters traveled in a transect using the direct and indirect methods of sampling; for camera-trapping, unit of effort corresponds to the hectares covered.
We applied a Chi-square test at a confidence level of 95% using the program R (R Core Team 2018) to determine differences in the relative abundances between the disturbed and undisturbed zones. Furthermore, we evaluated sampling effort with a species accumulation curve using the Chao 2 non-parametric estimator in the software EstimateS (Colwell 2013). This index considers the number of expected species, evaluating the relationship between the number of unique species (that only appear in one sample) and the number of species that appear in two samples, and is considered the most rigorous and least biased for small samples (Villareal et al. 2006).
Finally, we compared the species recorded on Isla Santay with reports from several conservation areas in coastal provinces of Southwestern Ecuador (Parker & Carr 1992, Fundación Natura 2006, Fundación Bioeducar 2008, Lizcano et al. 2016, Cervera et al. 2016, Espinoza et al. 2016, Salas & Vera 2017, Vera & Salas 2020, Saavedra et al. 2017, Zambrano et al. 2019, García-Olaechea et al. 2021, Merchán 2021).
Results
We registered six species using direct sampling and three species using indirect sampling, with a total sampling effort of 30 hours in 90 days (Table 1, Fig. 3). The ocelot (Leopardus pardalis) showed the highest RAI for both zones, followed by the crab-eating raccoon (Procyon cancrivorus) (Fig. 4, Table 1).

Figure 3 Mammal species registered on Isla Santay: Odocoileus virginianus peruvianus, adult male (A), footprint (B), and scat (C); Leopardus pardalis: adult (D), footprint (E), and scat (F); Procyon cancrivorus: individual (G) and footprint (H); Tamandua mexicana (I); Lontra longicaudis: two individuals exhibiting mating behaviour (J) and footprint (K); Philander melanurus (L). Photos by ATD (B, C, E, F, and H), Edgar Vera (J) and JAS (K).
Table 1. Relative abundance index (RAI) of medium and large-sized mammals by zones and type of record on Isla Santay from August 2018 to January 2019.
| Species | Zones | Record | Number of events | RAI | |
|---|---|---|---|---|---|
| 1 | Philander melanurus | Disturbed | Direct observation | 1 | 0.2 |
| 2 | Tamandua mexicana | Undisturbed | Direct observation | 1 | 0.1 |
| Disturbed | Direct observation | 3 | 0.6 | ||
| 3 | Leopardus pardalis | Disturbed | Direct observation | 6 | 1.1 |
| Footprints | 3 | 0.6 | |||
| Scats | 6 | 1.1 | |||
| Undisturbed | Direct observation | 2 | 0.2 | ||
| Footprints | 10 | 1.2 | |||
| Scats | 18 | 2.2 | |||
| Scraper | 1 | 0.1 | |||
| 4 | Procyon cancrivorus | Undisturbed | Footprints | 3 | 0.4 |
| Scats | 2 | 0.2 | |||
| Disturbed | Direct observation | 4 | 0.7 | ||
| Footprints | 5 | 0.9 | |||
| Scats | 3 | 0.6 | |||
| 5 | Lontra longicaudis | Undisturbed | Direct observation | 2 | 0.2 |
| Disturbed | Direct observation | 6 | 1.1 | ||
| 6 | Odocoileus virginianus peruvianus | Undisturbed | Footprints | 2 | 0.2 |
| Scats | 1 | 0.1 |
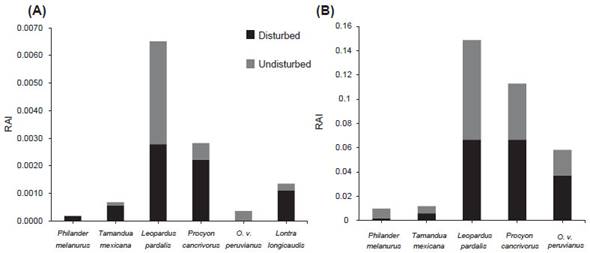
Figure 4 Relative abundance indices (RAI) of medium and large-sized mammals on Isla Santay from August 2018 to January 2019. (A): via direct and indirect sampling methods; (B): via camera-trapping.
We obtained 930 photos, corresponding to 363 independent photographic detections of five species of medium and large-sized mammals in both zones, with a sampling effort of 990 camera days. Leopardus pardalis presented the highest number of photographic events (Fig. 4), with 137 events in eight cameras, followed by Procyon cancrivorus with 111 events in 11 cameras, Odocoileus virginianus peruvianus with 54 events in seven cameras, Philander melanurus with 14 events in two cameras, and Tamandua mexicana with 13 events in seven cameras. In addition, we recorded four introduced species: Equus caballus presented the highest number of events with 141 events, followed by Bos taurus with 12 events, Sus scrofa domesticus with six events, and Canis lupus familiaris with four events; these data were not included in our statistical analysis.
The relative abundances of mammals between the disturbed and undisturbed zones were significantly different (X2 = 9.6404, df = 4, p = 0.0469). The species accumulation curve showed an asymptote at 20 days of sampling (Fig. 5). The species Philander melanurus and Lontra longicaudis were reported for the first time on Isla Santay (Table 2).
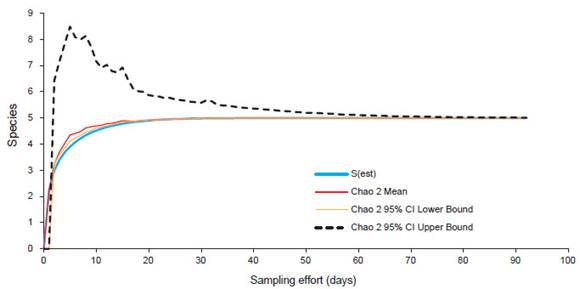
Figure 5 Species accumulation curve for medium and large-sized mammals on Isla Santay from August 2018 to January 2019.
Table 2. Annotated list of medium and large mammals recorded on Isla Santay from August 2018 to January 2019, compared with other conservation areas in coastal provinces of Southwestern Ecuador. Manabí: RVA= Refugio Valle Alto (Zambrano et al. 2019), P= Refugio de Vida Silvestre Marino Costero Pacoche (Lizcano et al. 2016); M= Parque Nacional Machalilla (Parker & Carr 1992, Cervera et al. 2016); Los Ríos: J= Bosque Protector Pedro Franco Dávila o Jauneche (Parker & Carr 1992, Salas & Vera 2017); AdM= Humedal Ramsar Abras de Mantequilla (Vera & Salas 2020); Guayas: MES= Reserva de Producción de Fauna Manglares El Salado (Fundación Natura 2006, Fundación Bioeducar 2008), CB= Bosque Protector Cerro Blanco (Saavedra et al. 2017, Merchán 2021), IS= Área Nacional de Recreación Isla Santay y del Gallo (present study); El Oro: A= Reserva Ecológica Arenillas (Espinoza et al. 2016, García-Olaechea et al. 2021), PP = Bosque Protector Petrificado Puyango (Merchán 2021). Conservation status follows the IUCN and Ecuadorian (E) Red Lists (Tirira 2021). Type of record: C= camera trap, O= direct observation, T= tracks, and I= interview. An asterisk (*) references a dead individual found, a plus sign (+) indicates a historical record without confirmation of the exact date.
| Species | Manabí | Los Ríos | Guayas | El Oro | Conservation status | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORDER / Family | RVA | P | M | J | AdM | MES | CB | IS | A | PP | IUCN (E) | ||||
| NATIVE | |||||||||||||||
| DIDEPHIMORPHIA / Didelphidae | |||||||||||||||
| Philander melanurus (Thomas, 1899) | - | - | - | - | - | O | - | C | C | - | NE (LC) | ||||
| Caluromys derbianus (Waterhouse, 1841) | - | - | I | O+ | - | - | - | - | - | - | LC (VU) | ||||
| Didelphis marsupialis (Linnaeus, 1758) | C | C | C | O, T | O | O | C | - | C | C | LC (LC) | ||||
| Metachirus myosuros (Temminck, 1824) | - | - | - | - | O | O | - | - | - | - | LC (LC) | ||||
| ARTIODACTYLA / Cervidae | |||||||||||||||
| Odocoileus virginianus peruvianus (Zimmermann, 1780) | - | C | C | - | T | - | C, T, O | C | C | C | LC (EN) | ||||
| Mazama gualea J. A. Allen, 1915 | - | - | C | T+ | - | - | - | - | - | C | NE (EN) | ||||
| Tayassuidae | |||||||||||||||
| Dicotyles tajacu (Linnaeus, 1758) | - | - | C | - | - | - | C, T, O | - | C | - | LC (NT) | ||||
| RODENTIA / Dasyproctidae | |||||||||||||||
| Dasyprocta punctata Gray, 1842 | C | C | C | T+ | - | - | C, O, T | - | - | - | LC (LC) | ||||
| Cuniculidae | |||||||||||||||
| Cuniculus paca (Linnaeus, 1766) | C | C | C | T+ | - | - | C | - | C | C | LC (NT) | ||||
| LAGOMORPHA / Leporidae | |||||||||||||||
| Sylvilagus daulensis J. A. Allen, 1914 | C | C | C | - | O | T/O | C, O, T | - | C | - | NE (NT) | ||||
| CINGULATA / Dasypodidae | |||||||||||||||
| Dasypus novemcintus Linnaeus, 1758 | C | C | C | O | T | - | O | - | - | C | LC (LC) | ||||
| CARNIVORA / Felidae | |||||||||||||||
| Leopardus pardalis pusaeus Thomas, 1914 | C | C | C | T/O | I | - | C, T | C | C | - | LC (VU) | ||||
| Leopardus wiedii (Schinz, 1821) | C | C | C | - | - | - | - | - | - | C | NT (NT) | ||||
| Herpailurus yagouaroundi panamensis (J. A. Allen, 1904) | C | C | C | - | - | O | C | - | O*, C | - | LC (VU) | ||||
| Puma concolor (Linnaeus, 1771) | - | - | - | - | - | - | - | - | C, I | C | LC (EN) | ||||
| Panthera onca (Linnaeus, 1758) | - | - | - | - | - | - | T, C | - | - | - | NT (EN) | ||||
| Mustelidae | |||||||||||||||
| Lontra longicaudis annectens Major, 1897 | - | - | - | - | O | - | - | O | - | - | NT (EN) | ||||
| Galictis vittata (Schreber, 1776) | - | C | - | - | - | - | O | - | C | - | LC (DD) | ||||
| Eira barbara (Linnaeus, 1758) | C | C | C | - | - | - | C | - | C | C | LC (LC) | ||||
| Procyonidae | |||||||||||||||
| Procyon cancrivorus aequatorialis J. A. Allen 1915 | C | C | C | T | O | O, T | C, O, T | C | C | C | LC (NT) | ||||
| Potos flavus modestus Thomas, 1902 | - | - | C | O+ | O* | - | I | - | - | - | LC (VU) | ||||
| Nasua nasua (Linnaeus, 1766) | C | - | C | O+ | O | - | O, C | - | C | C | LC (NT) | ||||
| Canidae | |||||||||||||||
| Lycalopex sechurae (Thomas, 1900) | C | - | - | - | - | - | - | - | C, O | C | NT (EN) | ||||
| PILOSA / Myrmecophagidae | |||||||||||||||
| Tamandua mexicana (Saussure, 1860) | C | C | C | O, T | - | O | C, O | C | C | - | LC (EN) | ||||
| Bradypodidae | |||||||||||||||
| Bradypus variegatus ephipiger Philippi, 1870 | O | - | - | - | O | - | - | - | - | - | LC (EN) | ||||
| PRIMATES / Atelidae | |||||||||||||||
| Alouatta palliata (Gray, 1849) | O | C | C | O | O | - | O | - | - | - | VU (CR) | ||||
| Cebidae | |||||||||||||||
| Cebus aequatorialis J. A. Allen, 1914 | - | C | C | O | - | - | O | - | - | - | CR (CR) | ||||
| EXOTIC | |||||||||||||||
| Bos taurus Linnaeus, 1758 | - | C | - | - | - | - | - | C | - | - | N/A | ||||
| Sus scrofa Linnaeus, 1758 | - | C | - | - | - | - | - | C | - | - | N/A | ||||
| Canis lupus familiaris Linnaeus, 1758 | - | C | - | - | - | - | - | C | C | C | N/A | ||||
| Equus caballus Linnaeus, 1758 | - | C | - | - | - | - | - | C | - | - | N/A | ||||
| Equus asinus Linnaeus, 1758 | - | C | - | - | - | - | - | - | - | - | N/A | ||||
| Capra hircus Linnaeus, 1758 | - | C | - | - | - | - | - | - | - | - | N/A | ||||
| Felis silvestris catus Schreber 1775 | - | C | - | - | - | - | - | - | - | - | N/A | ||||
Discussion
In a previous survey, 14 species of medium and large-sized mammals were reported for Isla Santay by interviews with local inhabitants and direct observations (MAE 2011). In our investigation, we confirmed the presence of four species: Odocoileus virginianus peruvianus, Leopardus pardalis, Procyon cancrivorus, and Tamandua mexicana, and added Philander melanurus and Lontra longicaudis as new records for this protected area. Despite the adequate sampling effort, several of the species previously reported by MAE (2011) were not recorded, such as didelphids (Didelphis marsupialis, Marmosa simonsi), carnivores (Nasua nasua, Potos flavus, Eira barbara, Galictis vittata, Herpailurus yaguarondi), and primate species. Several of these species have a broad geographic range and have been reported in wetlands and other nearby conservation areas in Western Ecuador (Briones et al. 2001, Cervera et al. 2016, Espinoza et al. 2016, García-Olaechea et al. 2021, Lizcano et al. 2016, Saavedra et al. 2017, Zambrano et al. 2019, Solórzano et al. 2021).
The absence of these species could indicate local extirpation, potentially caused by a synergistic combination of the loss and fragmentation of habitat, hunting, introduced species, and illegal trade (Tirira 2011). Recruitment of new individuals may have not occurred due to the geographic isolation of this protected area, since it is surrounded by urban centers with progressive demographic growth, restricting movement and genetic exchange with other mammal populations. Nevertheless, the confirmed presence of carnivores on Isla Santay is important since they play key roles in their ecosystems (Rumiz 2010); their presence can be attributed to their ability to adapt to landscape changes and live in several different environments. For example, Procyon cancrivorus is present in peri-urban areas, deciduous and wet forests, streams, and swamps across many coastal conservation areas (Briones et al. 2001, Salas & Vera 2017, Saavedra et al. 2017, Cervera et al. 2016, Lizcano et al. 2016, Espinoza et al. 2016, Vera & Salas 2020); currently, the populations from coastal Ecuador are designated as P. c. aequatorialis J. A. Allen, 1915, and are categorized as Near Threatened (NT) (Tirira 2021).
One of these carnivores, the ocelot Leopardus pardalis, occupies a broad variety of habitats and environments: protected areas, buffer zones, farms, private properties, and small remnant patches of native forest (Zambrano et al. 2019, Lizcano et al. 2016, Espinoza et al. 2016, Cervera et al. 2016, Salas & Vera 2017). The large number of photographic events obtained for L. pardalis is remarkable and may be a consequence of movement behaviour patterns in a small protected area, so it is necessary to identify individuals and calculate the population density; thus, future long-term studies utilizing double the number of camera-trapping stations (Pérez-Irineo & Santos-Moreno 2014) might allow a better population assessment. Before Isla Santay was declared a protected area, the inhabitants hunted ocelots to prevent them from preying on poultry (MAE 2011). Fortunately, human-carnivore conflict has been mostly eliminated due to increased levels of domestic animal management and care by the island’s inhabitants (A. Torres-Domínguez pers. obs.). Therefore, a change in perception was observed once Isla Santay became a protected area. Currently, L. pardalis is categorized as Near Threatened (NT) in Ecuador due to habitat loss, illegal trade, and conflicts with humans; the population from the mid-southern region of coastal Ecuador is classified as L. p. pusaeus and categorized as Vulnerable (VU) (Tirira 2021).
Besides the ocelot, three more species registered on Isla Santay are included in the Red List of Mammals in Ecuador. One of this is the White-tailed deer, Odocoileus virginianus peruvianus, categorized as Endangered (EN) and threatened by deforestation, habitat reduction, and attacks by feral dogs (Tirira 2021). During our investigation, young males of O. v. peruvianus (up to three years of age) were observed to be active both during the day and at night; this is consistent with a report from Bosque Protector Cerro Blanco (Merchán 2021), located 15 km from Isla Santay, and confirms that this species manifests a cathemeral behavior (Cortés-Marcial & Briones-Salas 2014). The white-tailed deer presented a higher RAI for camera-trapping events in the undisturbed zone (0.037) than the disturbed (0.02). The lower RAI in the disturbed zone may be the result of tourism and anthropic activities or competition with introduced species (cows, horses, and goats) that induce its displacement to the undisturbed zone (Flores-Armillas et al. 2011). Future long-term studies should also examine the negative effect of introduced or exotic faunas on native mammals in this reserve.
Another recorded threatened species was the Neotropical otter Lontra longicaudis, categorized as Vulnerable (VU) and Near Threatened (NT) on the Ecuadorian and IUCN Red Lists, respectively (Tirira 2021, Rheingantz et al. 2021). Agriculture, aquaculture, the expansion of urban areas, and the pollution of aquatic habitats are the principal threats that L. longicaudis faces, and an information gap exists in the coastal region of Ecuador regarding the ecology of the species and the impact of these threats on its populations (Utreras et al. 2011); according to Tirira (2021), the populations of coastal Ecuador are classified as L. l. annectens Major, 1897, and are categorized as Endangered. Recent ecotoxicological studies have reported high concentrations of cadmium, mercury, and lead in various locations of the Guayas River basin, including Isla Santay (Mero et al. 2019, Navarrete-Forero et al. 2019, Pernía et al. 2019), so it is necessary to determine if these transition metals are bioaccumulating in L. longicaudis and identify possible genotoxic effects of these elements in L. longicaudis populations (Walker et al. 2012).
Previously, the Neotropical otter had not been recorded as present in the SNAP protected areas in Western Ecuador (Fundación Bioeducar 2008, Cervera et al. 2016, Espinoza et al. 2016, Lizcano et al. 2016) and has only been confirmed as present in Abras de Mantequilla (Vera & Salas 2020), despite the broad distribution of this species in the region (Larivière 1999, Tirira 2017). The apparent lack of records of Lontra longicaudis in coastal-marine protected areas are most likely due to the fact that previous studies were focused on terrestrial habitats, whereas the records herein presented occurred in mangroves and flooded areas. It is necessary to confirm its presence in other protected areas and estimate the population size as well as habitat and diet preferences (Restrepo & Botero-Botero 2012, Lavariega et al. 2020), since ecological information regarding this species is very scarce and outdated.
Finally, the northern tamandua Tamandua mexicana presented a higher frequency of camera-trapping events in the undisturbed zone than in the disturbed zone, concurring with Navarrete & Ortega (2011), who indicated that this species inhabits deciduous tropical forest, mangroves, and second-growth forest. It is probable that these habitats provide an insect-based diet (such as ants and termites) and arboreal resources including limbs or lianas which tamanduas utilize for mobility; human structures may hinder its colonization or dispersal processes (Nuñez-Pérez et al. 2011). This species is categorized as Endangered (EN) due to habitat loss, hunting, attacks by dogs, and wildlife-vehicle collisions (Tirira 2021), but these threats are not present on Isla Santay today, making the reserve an ideal place to protect this and other threatened species. Nevertheless, the geographic isolation of this reserve prevents the recruitment of individuals from contiguous areas, so it is necessary to estimate the long-term population viability of these subpopulations. Future research should focus on improving ecological connectivity to other protected areas in the Gulf of Guayaquil (Mandujano-Rodríguez 2012).
The mammal richness found on Isla Santay was low compared to other large conservation areas in Western Ecuador (Cervera et al. 2016, Lizcano et al. 2016, Espinoza et al. 2016, Saavedra et al. 2017, García-Olaechea et al. 2021, Solórzano et al. 2021, Salas & Vera 2017, Vera & Salas 2020). However, the large number of visitors that the island receives provides an opportunity for environmental education to help to protect species at risk of extinction (MAE 2018), and its nearness to the city would facilitate logistics for the development of ecological studies of population size or diet. Isla Santay is a key conservation area for medium and large-sized mammals as well as threatened species, providing a refuge for this fauna on the Ecuadorian coast, and should especially be prioritized because of its small size and isolated location between expanding urban centers.












 uBio
uBio 


