Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Medica Herediana
versión impresa ISSN 1018-130X
Rev Med Hered vol.27 no.1 Lima ene. 2016
REPORTE DE CASO
Urinary rhabditiform larvae of Strongyloides stercoralis in disseminated disease affecting a kidney-transplanted patient
Larvas rabditoides de Strongyloides stercoralis en orina en paciente con riñón trasplantado y estrongiloidiasis diseminada
Leonor Pocaterra 1,a, Gladymar Pérez 1,b, Elsy Rojas 1,c, Aurora Hernán 1,c, Julio Castro 2,d, Carlos Goldstein 3,e, Luz Núñez 1,f
1 Parasitology Department.,Escuela de Medicina José María Vargas, Facultad de Medicina, Universidad Central de Venezuela. Caracas, Venezuela.
2Instituto de Medicina Tropical Dr. Félix Pifano C., Facultad de Medicina, Universidad Central de Venezuela. Caracas, Venezuela.
3Centro Médico de Caracas. Caracas, Venezuela.
a MD, DTM&H, MSc TM&H;
b MD, Paediatrician and Infectious Diseases specialist;
c Bionalyst;
d MD, Physician and Infectious Diseases specialist;
e MD, Physician and Haematologist;
f MD, Hematologist. MSc Parasitology.
RESUMEN
Strongyloides stercoralis, nemátode intestinal de alta prevalencia en zonas tropicales y subtropicales, se presenta clínicamente de forma asintomática u oligosintomática en personas inmunocompetentes. No obstante, la diseminación o hiperinfección por este parásito, puede producir severas complicaciones, a veces fatales en pacientes inmunosuprimidos. Este reporte describe a un paciente masculino de 53 años, trasplantado renal, cuyas complicaciones coinciden con la detección de larvas en las heces. La terapia inmunosupresora usual no impidió el desarrollo de nefritis aguda en este paciente. Los métodos de Baermann y cultivo en agar resultaron positivos en tres muestras consecutivas. Adicionalmente, en el sedimento urinario, se encontraron larvas rabditoides. Posterior al tratamiento con ivermectina la función renal evidenció significativa mejoría. El caso presentado enfatiza la necesidad de investigar la infección por S. stercoralis en los protocolos pre-trasplante en donantes y receptores, y en las complicaciones post-trasplante asociadas con eosinofilia, especialmente cuando proceden del trópico.
PALABRAS CLAVE: Strongyloides stercoralis, trasplante, orina. (Fuente: DeCS BIREME).
SUMMARY
Strongyloides stercoralis, an intestinal nematode prevalent in tropical and subtropical zones, remains clinically silent or mildly symptomatic in immunecompetent individuals. In contrast, clinical dissemination and/or hyperinfection may induce severe widespread damage and fatal outcomes in immunecompromised hosts. This report describes a 53-year-old male renal transplant recipient, in whom standard immunosuppressive therapy did not prevent development of acute nephritis also coinciding with appearance of larvae in fecal smears. During a 3-days sequential copro-parasitological testing S. stercoralis larvae were identified in Baermann and agar culture tests simultaneously with rhabditiform larvae of S. stercoralis in his urinary sediment. Significant improvement of renal dysfunction with ivermectin therapy highlights the importance of incorporating S. stercoralis screening in pre-transplant protocols and post-transplant complications.
KEYWORDS: Strongyloides stercoralis, transplantation, urine. (Source: MeSH NLM).
INTRODUCTION
Strongyloidiasis is an endemic soil-transmitted helminthiasis, common in tropical and subtropical areas, caused by Strongyloides stercoralis, an intestinal nematode capable of surviving for years in humans by perpetuating its life cycle through autoinfection. Recent reviews, perhaps reflecting improved and extensive availability of diagnostic tools, postulate human strongyloidiasis in approximately 370 million (1,2).
Most immunecompetent hosts remain clinically silent or only mildly symptomatic. Those immune compromised by serious infections (HIV/AIDS, HTLV-1)(3), hematological or solid organ malignancies, malnutrition, transplants or by immune-suppressive therapy are most susceptible to severe complications. Typically, intense manifestations (mainly cutaneous and gastrointestinal) also exhibiting marked eosinophilia, if untreated, can evolve into a multiorgan hyperinfection syndrome carrying a high fatality rate (>50%) (4,5). Consequently, sensitive screening procedures should be routinely employed to explore pre-transplant donors and all recipients in endemic regions, where parasitic infections remain generally underdiagnosed.
CASE REPORT
A 53-year-old HIV-negative kidney-transplanted male, affected by intermittent diarrhea and hypogastric pain, was referred to our clinic after larvae were observed on direct fecal smears. Transplantation had been performed 4 months earlier. Initial rejection prophylaxis with prednisone (50 mg/day for 15days) was followed by decreasing doses and subsequent replacement with mycophenolate mofetil and sirolimus. Ancillary anti-hypertensive and anti-hyperlipemia medications were maintained.
Epidemiologically, he had bathed in western Venezuelan rivers and beaches and had practiced frequent plant sowing for the last 10 years. Four years pre-transplantation he noticed a self-limited non-pruriginous papular rash localized to face and back, and 6 months pre-procedure he noticed epigastric pain and intermittent diarrhea. Pertinent findings included anemia, mild eosinophilia (Table 1), and active erosive gastritis with diffuse edema and vascular congestion of the lamina propia.
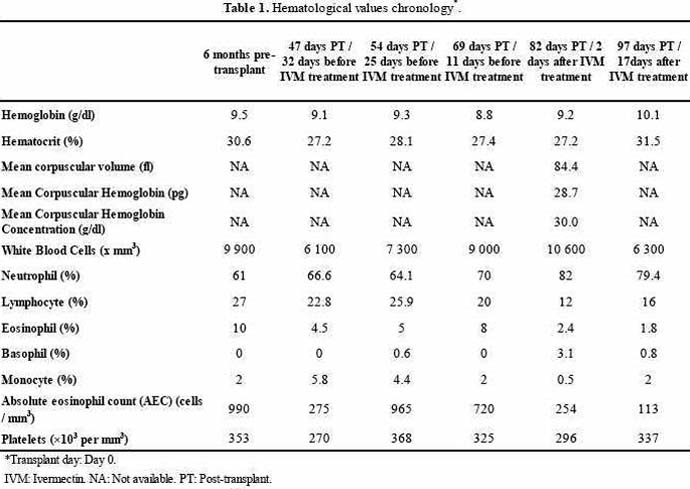
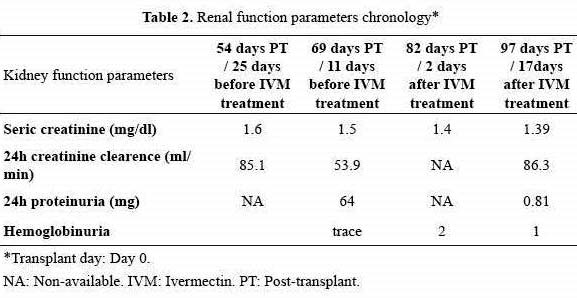
Coprologic screening for strongyloidiasis was not undertaken. At one month post-transplant he had lost 5 kg, and developed a lower limb edema and a 4-week lasting dry cough. Hemoglobinuria, proteinuria and decreased creatinine clearance coincided with normal albuminemia (Table 2).
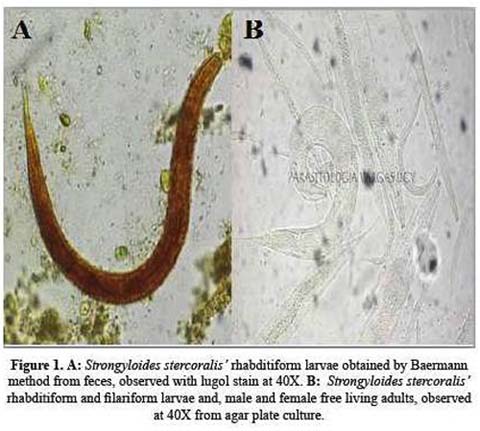
Over 100 rhabditiform larvae per field were revealed by Baermann’s method, some also appearing simultaneously in urinary sediments. Three fecal agar cultures further confirmed filariform larvae (Figure 1).
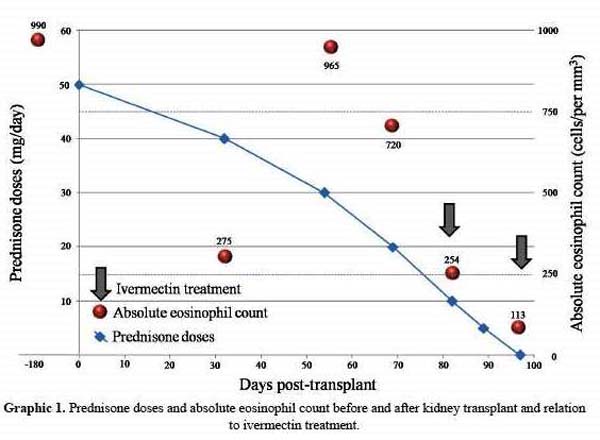
After discontinuing all immunosuppressive medications 2 doses of ivermectin (200 µg/kg per dose) were administered in a 7 day interval. Rapid improvement of clinical and renal function ensued, eosinophil counts normalized after 17 days (Table 1 and graphic 1) and agar cultures and urinary sediments turned larvae-negative. He did not return for a direct follow up but on a telephone interview he denied any symptom.
DISCUSSION
Undiagnosed or late recognition in inmunecompromising illnesses may preclude a timely identification of accelerated autoinfection, hyperinfection and dissemination of Strongyloides stercoralis as well as polymicrobial sepsis, determining common fatal outcomes. In endemic areas, like Venezuela, probing for chronic infections should probably be extended to most asymptomatic individuals. Current guidelines recommend serological screening or selective stool examinations in all pre-transplantation high-risk patients and after the procedure a high level of suspicion to prevent hyperinfection syndromes (6). Of note, in our propositus digestive manifestations and eosinophilia were initially ignored.
During chronic steroid administration, S. stercoralis spread is a well-known complication, mainly highlighted in renal transplants, but not unusual in solid organ and bone marrow recipients (7). As to other anti-rejection drugs, linkage of infection and dissemination to sirolimus remains unproven, although in a fatal bone marrow transplant recipient it was added to prednisone and mycophenolate mofetil (6).
Previous observations implicated S. stercoralis invasions in immunecompromised hosts as culprits of acute nephritis (6,7). Our patient’s renal and urinary abnormalities, and his recovery after ivermectin therapy, implies a parasite’s pathogenic role in inducing glomerular damage, possibly through immunological reactions involving parasitic antigens (8). A similar immune mechanism has been assumed in rare cases of arthritis in which a reactive mechanism has been suggested, although Strongyloides larvae had been encountered in synovial biopsies, as a possible alternative (9). Fortunately such secondary lesions, including the ones of our case, recovered fully after anthelmintic treatment.
Interestingly, urinary rhabditiform and filariform undocumented stages of S. stercoralis larvae are not uncommon in both, transplanted and non-transplanted hyperinfected renal patients (4,10-15). Presence of only rhabditiform larvae in our case presumes an intrarenal special autoinfection cycle in which filariform larvae develop into adult forms (parthenogenic females). This leads to ova production originating rhabditiform larvae expelled in the urine. Adults, eggs and rhabditiform larvae have also been recovered from tracheo-bronchial secretions (1,16-19).
Several difficulties preclude a more widespread diagnosis of strongyloidiasis. Copro-parasitological methods require well-trained personnel, scarce in most affected demographic areas, hampering even adequate handling of serial stool samples. If it becomes available, accurate serological testing or PCR would minimize these adversities, benefit many clinical needs such as transplant-related cases, and facilitate widespread epidemiological screening. As observed in our propositus, 1-day weekly ivermectin for 2 weeks offers similar high cure rates as conventional 2-day doses, also administered twice. Either way additional dosing is required in high-risk or relapsed cases which should be supervised periodically for at least a year.
REFERENCES
1. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013; 7(5):e2214. [ Links ]
2. Krolewiecki AJ, Lammie P, Jacobson J, et al. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis. 2013; 7(5):e2165. [ Links ]
3. Barros N, Risco J, Rodríguez C, et al. CD4+ T cell subsets and Tax expression in HTLV-1 associated diseases. Pathog Glob Health. 2013; 107(4):202-6. [ Links ]
4. Centers for Disease Control and Prevention (CDC). Transmission of Strongyloides stercoralis through transplantation of solid organs-Pennsylvania, 2012. MMWR Morb Mortal Wkly Rep. 2013; 62(14):264-6. [ Links ]
5. Núñez-Sifonte L. Estrongiloidosis: aspectos clínicos, hematológicos e inmunológicos. Med interna. 2000; 16(2):108-17. [ Links ]
6. Le M, Ravin K, Hasan A, et al. Single donor- derived Strongyloidiasis in three solid organ transplant recipients: Case series and review of the literature. Am J Transplantat. 2014; 14(5):1199-1206. [ Links ]
7. Izquierdo I, Briones J, Lluch R, Arqueros C, Martino R. Fatal Strongyloides hyperinfection complicating a gram-negative sepsis after allogeneic stem cell transplantation: a case report and review of the literature. Case Rep Hematol. 2013; 2013:860976. [ Links ]
8. Miyazaki M, Tamura M, Kabashima N, et al. Minimal change nephrotic syndrome in a patient with strongyloidiasis. Clin Exp Nephrol. 2010; 14(4):367- 71. [ Links ]
9. van Kuijk AW, Kerstens PJ, Perenboom RM, Dijkmans BA, Voskuyl AE. Early-onset polyarthritis as presenting feature of intestinal infection with Strongyloides stercoralis. Rheumatology (Oxford). 2003; 42(11):1419-20. [ Links ]
10. Mitsunaga M, Miyauchi N, Akiyama Y, Saima S. A case of strongyloidiasis hyperinfection during oral corticosteroid therapy associated with a nephrotic patient infected with HTLV-1. Nihon Ronen Igakkai Zasshi. 2003; 40(4):397-401. [ Links ]
11. Bangs MJ, Sirait S, Purnomo, Maguire JD. Strongyloidiasis with gastric mucosal invasion presenting with acute interstitial nephritis. Southeast Asian J Trop Med Public Health. 2006; 37(4):641-7. [ Links ]
12. Fowler CG, Lindsay I, Levin J, Sweny P, Fernando ON, Moorhead JF. Recurrent hyperinfestation with Strongyloides stercoralis in a renal allograft recipient. Br Med J (Clin Res Ed). 1982; 285 (6352):1394. [ Links ]
13. Hoy WE, Roberts NJ Jr, Bryson MF, et al. Transmission of strongyloidiasis by kidney transplant?: Disseminated strongyloidiasis in both recipients of kidney allografts from a single cadaver donor. JAMA. 1981; 246(17):1937-9. [ Links ]
14. Morgan JS, Schaffner W, Stone WJ. Opportunistic strongyloidiasis in renal transplant recipients. Transplantation. 1986; 42(5):518-24. [ Links ]
15. Pasqualotto AC, Zborowski MF, dos Anjos M, Poloni JA, dos Santos AP, Torelly AP. Strongyloides stercoralis in the urine. Trans R Soc Trop Med Hyg. 2009; 103(1):106-7. [ Links ]
16. Yu JJ, Lu SC, Wu IH, Yu MC, Lee CN, Lin TP. Disseminated Strongyloides stercoralis infection mimicking pneumonia. J Formos Med Assoc. 1995; 94(S2): 162-5. [ Links ]
17. Casati A, Cornero G, Muttini S, Tresoldi M, Gallioli G, Torri G. Hyperacute pneumonitis in a patient with overwhelming Strongyloides stercoralis infection. Eur J Anaesthesiol. 1996; 13(5):498-501. [ Links ]
18. Lemos LB, Qu Z, Laucirica R, Fred HL. Hyperinfection syndrome in strongyloidiasis: report of two cases. Ann Diagn Pathol. 2003; 7(2):87-94. [ Links ]
19. Schroeder L, Banaei N. Images in clinical medicine: Strongyloides stercoralis embryonated ova in the lung. N Engl J Med. 2013; 368(12): e15. [ Links ]
Declaration of conflict of interest:
This study was financed by the Consejo de Desarrollo Científico y Humanístico de la Universidad Central de Venezuela. The authors do not have any conflict of interest to declare. They all have participated in the study and concur with the submission and subsequent revisions of the manuscript.
Contribution of authorship:
LP: Patient’s attending physician in Parasitology outpatient clinic. Conceived and wrote the case report in Spanish. Edited the english version. Elaborated the graphs; GP: Attending physician in Parasitology outpatient clinic. Wrote the paper´s English version; ER: Performed strongyloidiasis diagnosis and detected urinary rhabditiform larvae; AH: Edited the paper´s English version. Elaborated the graphs; JC: Patient´s Infectious disease attending physician. He referred the patient to Parasitology outpatient clinic and laboratory to perform strongyloidiasis diagnosis; CG: Edited the paper´s final English version; LN: Attending physician in Parasitology outpatient clinic. Laboratory head. Edited the paper´s final English version.
Correspondence:
Leonor A. Pocaterra
Cátedra de Parasitología. Escuela de Medicina "José María Vargas". Edificio 2. Segundo piso. Facultad de Medicina. Universidad Central de Venezuela. Esquina de Pirineos. San José. Caracas 1010. Venezuela.
Telephone: 58-212-564-56-86. Fax: 58-212-562-99-28.
E-mail: leopoca@gmail.com
Recibido: 17/03/2015
Aceptado: 14/12/2015













