Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Gastroenterología del Perú
versión impresa ISSN 1022-5129
Rev. gastroenterol. Perú vol.35 no.4 Lima oct. 2015
Articulo de Revisión
Endoscopic-ultrasound versus percutaneous-guided celiac plexus block for chronic pancreatitis pain. A systematic review and meta-analysis
Bloqueo del plexo celiaco para el dolor por pancreatitis crónica – ultrasonido endoscópico versus percutánea: una revisión sistemática y meta-análisis
Renata Nobre Moura1, Eduardo Guimarães Hourneaux De Moura1, Wanderley Marques Bernardo1, Jose P. Otoch1, Fabio Alberto Castillo Bustamante1, Débora Vieira Albers1, Gustavo Luis Rodela Silva1, Dalton Marques Chaves1, Everson Luiz de Almeida Artifon1
1 Gastrointestinal Endoscopy Unit, University of São Paulo. São Paulo, Brazil
ABSTRACT
Background: Abdominal pain is present in the vast majority of patients with chronic pancreatitis, being frequently debilitating. Celiac plexus block (CPB) is an interventional technique that can be considered to provide a temporary pain relief. Objective: To estimate the effectiveness and safeness of endoscopic-ultrasound (EUS) comparing with percutaneous-guided CBP in patients with pancreatic pain. Methods: A systematic review of English and non-English articles using MEDLINE, EMBASE, LILACS and COCHRANE (via BVS). Study selection and data extraction: Only randomized control trials (RCT) comparing the beneficial and harmful effects of EUS and percutaneous-guided celiac plexus block for managing pancreatic pain were included. Data was extracted and analyzed on variables including pain relief and related procedure complications. Results: Two RCT met the inclusion criteria. Both studies assessed the primary outcome (reduction on pain score) and evaluated adverse effects. The drugs injected were the same; nevertheless percutaneous technique was guided by fluoroscopy in one study and by computer tomography (CT) in other. The results showed that the EUS-CPB group was more effective to reduce pain score after 4 weeks after the procedure, with risk of bias to do this affirmation. No statistical difference in pain relief at 1, 8 and 12 weeks and in complications rates. Conclusions: Based on this systematic review and meta-analysis, no statistically significant difference was noted in pain relief and complications for EUS and percutaneous - CPB.
Key words: Pancreatitis, chronic; Pain management; Celiac plexus; Endosonography; Meta-analysis (source: MeSH NLM).
RESUMEN
Antecedentes: El dolor abdominal es presente en la gran mayoría de pacientes con pancreatitis crónica, siendo con frecuencia debilitante. El bloqueo del plexo celíaco (BPC) es una técnica de intervención que puede ser considerado para proporcionar un alivio temporal del dolor. Objetivo: Estimar la eficacia y seguridad de la ecografía endoscópica-(EE) comparando con percutánea en pacientes con dolor de páncreas. Fuentes de datos: una revisión sistemática de los artículos utilizando MEDLINE, EMBASE, LILACS y COCHRANE (a través de la BVS). Selección de los estudios y la extracción de datos: se incluyeron solo ensayos controlados aleatorios que compararon los efectos beneficiosos y perjudiciales de la USE y bloqueo del plexo celiaco percutánea para el manejo del dolor de pancreas. Los datos fueron extraídos y analizados en variables incluyendo el alivio del dolor y las complicaciones de procedimientos relacionados. Resultados: Dos ensayos controlados cumplieron los criterios de inclusión. Ambos estudios evaluaron el resultado primario (reducción en la puntuación de dolor) y los efectos adversos. Las drogas inyectadas fueron las mismas; sin embargo, la técnica percutánea fue guiado por fluoroscopia en un estudio y por tomografía computarizada (TC) en el otro. Los resultados mostraron que el grupo de la EE fue más eficaz para reducir la escala de dolor después de 4 semanas del procedimiento, con el riesgo de sesgo de hacer esta afirmación. No hay diferencia estadística en el alivio del dolor en el 1, 8 y 12 semanas y en las tasas de complicaciones. Conclusiones: En base a esta revisión sistemática y meta-análisis, no se observaron diferencias estadísticamente significativas en el alivio del dolor y las complicaciones de la BCP por EE y percutánea.
Palabras clave: Pancreatitis crónica; Manejo del dolor; Plexo celíaco; Endosonografía; Metanálisis (fuente: DeCS BIREME).
INTRODUCTION
The management of pain associated with chronic pancreatitis (CP) continues to be a clinical challenge. Up to 85-90% of patients with CP have pain at the time of diagnosis, which may increase as the disease advances (1-3). Therefore, the optimal management of this symptom is important for improving the quality of live. Many methods have been proposed to treat these patients, including medical therapy (pharmacologic analgesics, pancreatic enzymes, octreotide, antioxidant agents), endoscopic therapy (ERCP procedures to drain the main pancreatic duct, removal of stones and dilation of strictures), celiac plexus block (by endoscopic, percutaneous or surgical techniques) and surgical approaches (thoracoscopic splanchnicectomy, intraoperative celiac plexus block, cryoablation and radiofrequency thermocoagulation, pancreas resections, etc.).
The exact mechanisms of pain are not fully understood. Many theories have been proposed to the pathogenesis of the abdominal pain associated with chronic pancreatitis and pancreatic cancer (4). Possible etiologies include celiac plexus invasion by tumor infiltration, pancreatic duct obstruction and distention, inflammation and ischemia (4,5). There appears to be significant cross talk among the different mechanisms, which could explain the partial or no success of single modality treatment approach (2).
Although pain can be well controlled with conventional analgesics, in some cases is difficult to treat or patients may suffer from drug-related side effects, such as constipation, nausea, vomiting, somnolence, confusion, and drug dependence and addiction (1,6). In these patients, interventional pain techniques may be indicated (7).
Celiac plexus block (CPB) has been used in the management of pancreatic pain since in 1914, when was first described by Kappis (1). CPB refers to the temporary inhibition of the celiac plexus often achieved with a corticosteroid injection in patients with benign pancreatic diseases like chronic pancreatitis. On the other hand, celiac plexus neurolysis (CPN) refers to the ablation of the plexus, often achieved with alcohol or phenol administered with a local anesthetic, such as bupivicaine which is injected first to prevent pain associated with the alcohol injection. CPN is not routinely used in benign diseases given the potential risks, such as retroperitoneal fibrosis, irreversible nerve injury and even paraplegia (3).
Several techniques to perform celiac plexus blockade have been published in the literature. Percutaneous-CPB can be carried out via a posterior or anterior approach, facilitated by computed tomography (CT) scan, fluoroscopy, or ultrasound (8). These techniques differ with respect to the route of needle insertion, use of radiologic guidance versus a blind procedure, and chemical composition of the injectate.
The advent of therapeutic EUS offers new options for management of pancreatic pain which, compared with the percutaneous approaches, has the theoretical benefits of enhancing needle localization and spread of the injectate, improving pain relief. More importantly, paraplegia has not been described after endoscopic ultrasonography-guided celiac plexus block (EUS-CPB), probably because of the anterior transgastric approach taken during endoscopic ultrasonography, decreasing or even eliminating the risk of nerve or spinal cord injury. Furthermore, EUS-CPB seemed to persist longer that CT-guided block (9). The current evidence indicates that this technique is safe and well tolerated, with excellent results.
This systematic review aims to evaluate the feasibility and effectiveness of EUS-CBP compared with percutaneous approach in patients with abdominal pain due to chronic pancreatitis, quantifying the effects of CPB on pain relief and to identify any adverse effects.
METHODS
Protocol and registration
This systematic review of the literature was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses) recommendations (10). The review was registered on PROSPERO international database (www.crd.york.ac.uk/prospero/ ) under number CRD42014014693.
Eligibility criteria
− Types of studies: Randomized clinical trials comparing EUS-guided and percutaneous technique CPB for CP pain control were included in this review.
− Types of participants: Participants of any age with pain due to CP were considered. CP was defined based on clinical and radiological features criteria.
− Types of intervention: EUS and percutaneous-guided celiac plexus block for managing pancreatic pain.
− Types of outcome measures: Primary outcome measure was pain relief based on a visual analogue scale (range: 0-10) before and after the procedure. Secondary outcome measure was complications related with the procedure.
− Studies about thoracoscopic splanchnicectomy (TS), intraoperative celiac plexus block, cryoablation and radiofrequency thermocoagulation were excluded. Studies comparing celiac plexus block with conventional pain management were also excluded.
Information sources
Articles were searched in electronic databases and scanning reference list. No limits were applied for language. This search was applied to Medline (1949–present), Lilacs and Cochrane (via BVS) (1975–present), and Embase (1980–present). The last search was run on November 2014.
Search
The search terms were intentionally broad and studies about CPB and CPN in patients with pancreatic cancer were initially included to ensure the review covered several aspects of the procedure. The objective of this approach was to detect as many papers as possible regarding CPN/CPB and pancreatic pain. The reference lists of all retrieved studies and the most recent review articles for percutaneous and EUS CPN was evaluated.
The search terms for each database were:
- Medline and Embase
("Celiac Plexus" OR "Plexus, Celiac" OR "Coeliac Plexus" OR "Plexus, Coeliac" OR "Plexus Coeliacus" OR "Coeliacus, Plexus" OR "Solar Plexus" OR "Plexus, Solar" OR "Splanchnic Nerve" OR "Nerve, Splanchnic" OR "Nerves, Splanchnic" OR "Block, Nerve" OR "Blocks, Nerve" OR "Nerve Blocks" OR "Nerve Blockade" OR "Blockade, Nerve" OR "Blockades, Nerve" OR "Nerve Blockades" OR "Chemical Neurolysis" OR "Chemical Neurolyses" OR "Neurolyses, Chemical" OR "Neurolysis, Chemical" OR "Chemodenervation" OR "Chemodenervations") AND ("Neoplasm, Pancreatic" OR "Pancreatic Neoplasm" OR "Pancreas Neoplasms" OR "Neoplasm, Pancreas" OR "Neoplasms, Pancreas" OR "Pancreas Neoplasm" OR "Neoplasms, Pancreatic" OR "Cancer of Pancreas" OR "Pancreas Cancers" OR "Pancreas Cancer" OR "Cancer, Pancreas" OR "Cancers, Pancreas" OR "Pancreatic Cancer" OR "Cancer, Pancreatic" OR "Cancers, Pancreatic" OR "Pancreatic Cancer" OR "Cancer of the Pancreas" OR "Carcinomas, Pancreatic Ductal" OR "Ductal Carcinoma, Pancreatic" OR "Ductal Carcinomas, Pancreatic" OR "Pancreatic Ductal Carcinomas" OR "Duct-Cell Carcinoma of the Pancreas" OR "Duct Cell Carcinoma of the Pancreas" OR "Pancreatic Ductal Carcinoma" OR "Ductal Carcinoma of the Pancreas" OR "Pancreatic Duct Cell Carcinoma" OR "Carcinoma, Ductal, Pancreatic" OR "Duct-Cell Carcinoma, Pancreas" OR "Carcinoma, Pancreas Duct-Cell" OR "Carcinomas, Pancreas Duct-Cell" OR "Duct Cell Carcinoma, Pancreas" OR "Duct-Cell Carcinomas, Pancreas" OR "Pancreas Duct-Cell Carcinoma" OR "Pancreas Duct-Cell Carcinomas" OR "Chronic Pancreatitis" OR "Pancreatitis")
- Cochrane and Lilacs (via BVS)
"Celiac plexus" OR "splanchnic nerve" AND "pancreatits" OR "cancer" OR "neoplasm"
Study selection
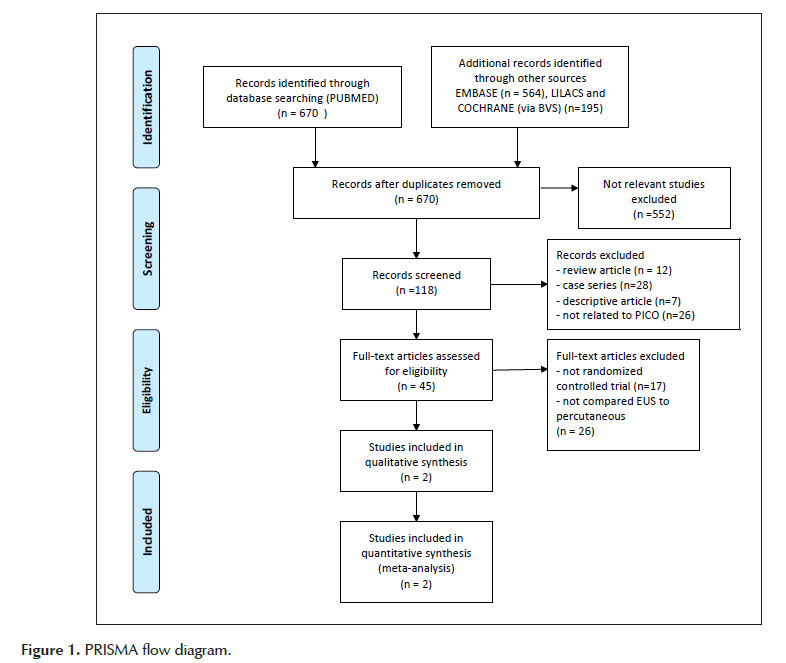
Two independent reviewers performed eligibility assessment and study selection. Any differences were resolved by mutual agreement. The final selection was summarized as a PRISMA flow diagram. (Figure 1)
Data collection process
Titles of papers were inspected and excluded if irrelevant. One review author extracted the following data from included studies and the other checked the extracted data. If there was doubt over whether an abstract should be included for full text retrieval, the decision was made to include.
It was used a worksheet form to summarize the data and to reduce bias and mistakes during the collection process. Since all the information necessary was available in the studies, it was not necessary to contact any authors.
Data items
Information extracted from each included: (1) characteristics of trial participants (number or patients, age, sex, diagnosis, use of pain medications, use of alcohol and baseline pain score, length of follow-up); (2) type of intervention (EUS or percutaneous-guided CPB, dose and type of substances injected, technique approach); (3) type of outcome measure (level of pain reduction, complications, overall experience and cost).
Risk of bias in individual studies
To ascertain the validity of eligible randomized trials, pairs of reviewers worked independently and with adequate reliability determined the adequacy of randomization and concealment of allocation, blinding of patients and extent of loss to follow-up.
JADAD criteria (11) was used to assess quality of the studies included in the meta-analyses. This criteria is used for critical analysis of an individual study. The validated score lies in the range 0-5. Studies are scored according to the presence of three key methodological features of randomization, blinding and accountability of all patients, including withdrawals. It was decided that studies should be scored as more consistent quality if they received a JADAD score of three or more (11).
Summary measures
The primary outcome measure was pain relief in 1, 4, 8 and 12 weeks after procedure. The visual analogue scale (VAS) pain score was used to evaluate treatment efficacy. To extract the data, pain scores before and after the CPB, as a continuous variable, were analyzed as difference in means and compared using the Wilconox rank sum test. A positive response was defined as a decrease in pain score of more than or equal to 3 points. The Kaplan-Meier method was used to compare the EUS and percutaneous techniques over time until the pain score returned to the pretreatment or baseline score. Data were manually extracted from the Kaplan-Meier’s graphics by drawing a vertical line in pre-determinated times (1, 4, 8 and 12 weeks), so the analysis could be performed using the critically appraised topic (CAT) software, computing the control event rate (CER), experimental event rate (EER), absolute risk reduction (ARR) and number needed to treat (NNT) of each outcome, with a confidence interval of 95%.
Planned methods of analysis
The software program Review Manager (RevMan) Version 5.3 was utilized to perform meta-analyses and to present the results graphically (forest and funnel plots). The effect of publication bias was assessed by evaluating a funnel plot of the trial mean differences for asymmetry, using Mantel- Haenszel statistical method.
A Chi2- based test of heterogeneity was performed using Cochran’s Review Manager statistic program and calculated I2, the percentage of the total variability in effect estimates among trials that is due to heterogeneity rather than chance. Combined means difference were calculated, and a two-sided p value < 0.05 indicated statistical significance. The inverse variance method was used to calculate the mean differences and 95% confidence intervals (CI) for pain relief. Heterogeneity was assessed using the c2 test (p <0.10 indicating significant heterogeneity) and the quantity of heterogeneity by the I2 statistic (substantial heterogeneity if I2 >50%). The meta-analysis was performed by computing relative risks (RRs) using fixed-effects model.
Risk of bias across studies
Before performing the statistical combination of the studies, the biases between them were evaluated. From a methodological point of view, the sources of heterogeneity among the studies are many: random, differences in design, the form of patient selection, differences in applied therapeutic interventions and others methodological differences. This statistical heterogeneity refers to differences between study results beyond those attributable to chance.
Heterogeneity was estimated by bias indicators and construction of funnel plots. If identified some study out of the graphic (outlier), then asymmetry may be due to reporting bias, so this study was excluded and done a new analysis, as explained below. If there are no outliers, it will be consider true heterogeneity.
Additional analyses
Sensitive analysis was done when significant heterogeneity was found (I2 >50%). In this situation, we first found out what factors might explain the meaningful discrepancies; sometimes we decided to withdraw the outliers detected on funnel plot graphic. This was done so the results of the review could be regarded with a higher degree of certainty. After the withdrawal, other forest and funnel plots will be generated so the results could change.
RESULTS
Study selection
An initial search identified 670 reference articles. Of these, 118 relevant studies were selected and reviewed. After screening the remaining titles and abstracts against inclusion/exclusion criteria, forty-five publications were included for further reading and comprehensive assessment. Twenty-three RCTs (12-35) that evaluated the effect of CPN/B on pain were identified, but only two of them compared EUS and percutaneous techniques, both in CP patients. These two studies were included in this meta-analysis. There were no nonrandomized comparative studies between the two techniques neither for pancreatic cancer nor for chronic pancreatitis.
Study characteristics
The studies finally selected for the review were randomized controlled trials published in English. All subjects were diagnosed and documented as chronic pancreatitis based on clinical features and documented by abdominal ultrasound, CT scan or retrograde cholangiopancreatography (ERCP) and confirmed by EUS during the celiac block. Patients were eligible if they presented with intractable abdominal pain that was not controlled by currently used therapies. The total number of patients was 74 and the etiology for CP was idiopathic in 75%. None of the subjects had any local complications of pancreatitis that could cause the abdominal pain.
Gress et al (27) RCT was conducted in the USA and the intervention received 10 ml of bupivacaine followed by 3ml of triamcinolone (40 mg), injected on both sides of the celiac plexus region using a transposterior approach and a sterile 22-gauge, 15 cm-long spinal needle. This needle was inserted under CT guidance.
Santosh et al (18) conducted the study in India and used 10 ml of bupivacaine followed by 3 ml of triamcinolone (40 mg) injected by the percutaneous fluoroscopy-guided technique, using a posterior approach with a 22-gauge, 17 cm-long spinal needle. This needle was inserted and advanced using the ‘walking off’ the vertebra technique and positioned two centimeters anterior to the upper border of the first lumbar vertebra in the antecrural space.
In both studies the primary outcome assessed was the reduction of pain score based on a VAS and evaluated adverse effects, including those of any kind and serious events. Cost analysis and patient overall experience were measured only in one study (27).
The follow-up was up to 24 weeks for both studies and included 74 participants. Decreases of pain and reduction in medication requirements were evaluated until return to pretreatment or baseline pain scores. A researcher who was not blinded to the intervention obtained the response, through a questionnaire applied for the patients.
Risk of bias within studies
As stated before, the risk of bias was assessed using JADAD criteria. Both studies were considered consistent, with a final score of 3. The blinding criteria was not scored as therapeutic trials like these are difficult or impossible to fit into the double-blind format.
Results of individual studies
The visual analogue scale (VAS) pain score was used to evaluate treatment efficacy and was compared pre and post procedure as difference in means. At the baseline, there were no differences in both the groups.
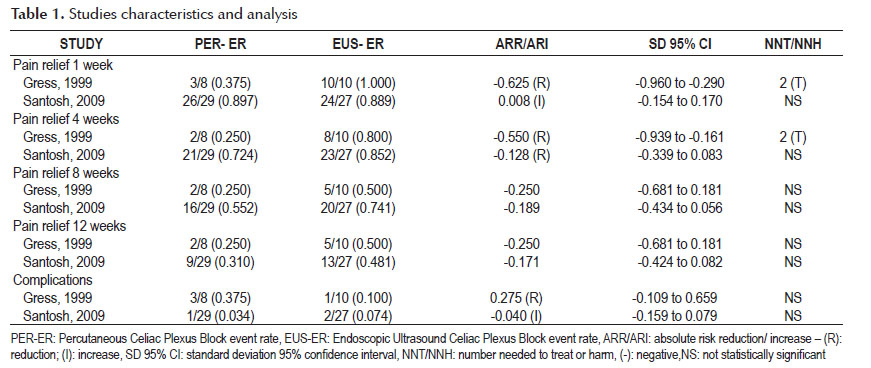
In general, patients treated with EUS-guided celiac plexus block had lower median post block pain scores when compared with scores in patients treated with percutaneous technique. Results of individual studies are shown in Table 1.
Syntheses of results
- Pain relief at 1 week (Figure 2).
Analysis of the pooled data revealed no differences in pain relief at the first week after EUS and percutaneous-CPB (95% CI: -0.01 to 0.30). The heterogeneity test indicated I2 =91%, demonstrating high heterogeneity. In the funnel plot analysis the study (Gress 1999), was identified as the cause of heterogeneity, and by consensus, the reviewers opted to withdraw this study from the meta-analysis, producing another forest plot. Exclusion of this study did not affect the finding of no evidence of difference in pain relief between the techniques (95% CI: -0.17, 0.15, p =0.93).
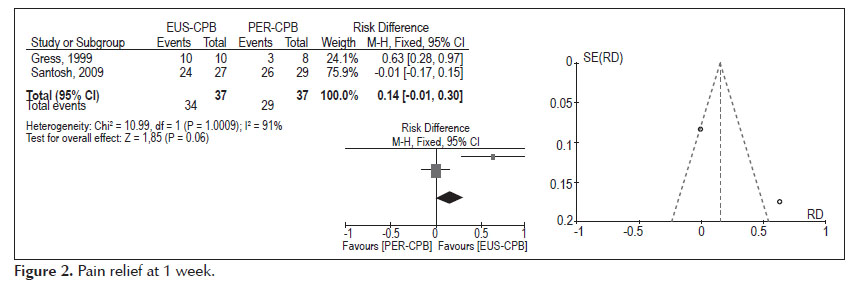
- Pain relief at 4 weeks (Figure 3).
Analysis of the pooled data revealed differences in pain relief at the fourth week in favours to EUS-CPB, with statistically significant results (95% CI: 0.04, 0.42, p =0.02). Nevertheless, the heterogeneity test indicated X2 =3.50 and I2 =71%, demonstrating high heterogeneity.
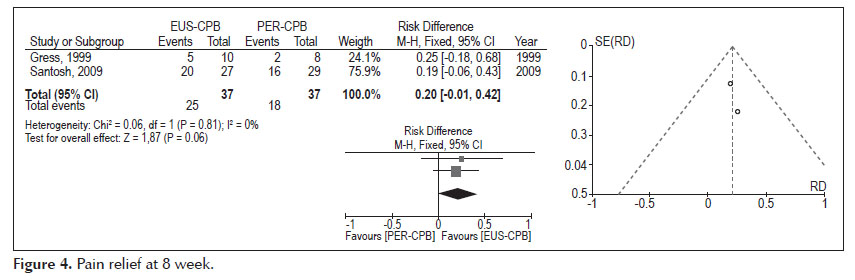
- Pain relief at 8 weeks (Figure 4).
Analysis of the pooled data revealed differences in pain relief at the eight week, in favors to the EUS therapy, affirmation with no statistical significance (95% CI: -0.01, 0.42, p =0.06). The heterogeneity test indicated X2 =0.06 and I2 =0%, demonstrating homogeneity.
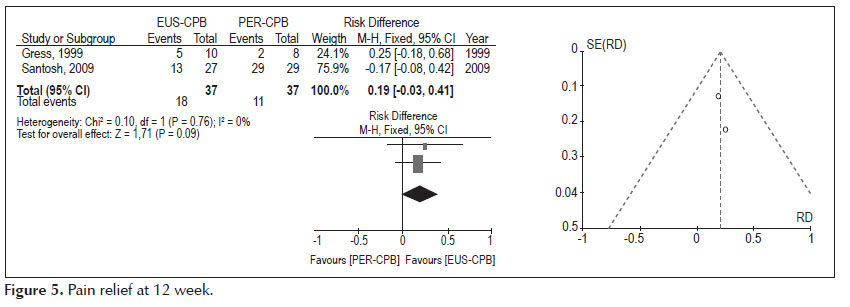
- Pain relief at 12 weeks (Figure 5).
Analysis of the pooled data revealed differences in pain relief at the twelfth week, in favors to the EUS therapy, affirmation with no statistical significance (95% CI: -0.03, 0.41, p =0.09). The heterogeneity test indicated X2 =0.10 and I2 =0%, demonstrating homogeneity.
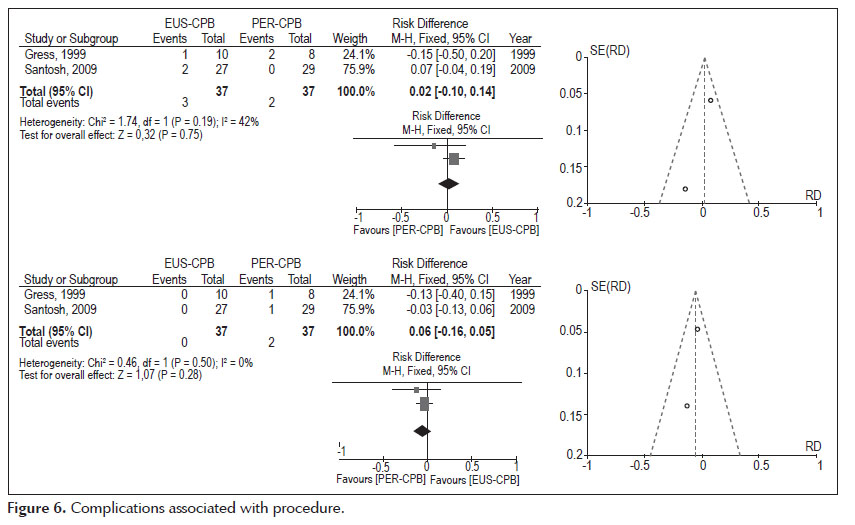
- Complications associated with procedure (Figure 6).
Diarrhea: Analysis of the pooled data revealed no differences in complications associated with procedure diarrhea, affirmation without statistical significance (95% CI: -0.10, 0.14, p =0.75). The heterogeneity test indicated X2 =1.74 and I2 =42%, demonstrating little heterogeneity.
Hypotension: Analysis of the pooled data revealed no differences in complications associated with procedure diarrhea, affirmation without statistical significance (95% CI: -0.16, 0.05, p =0.28). The heterogeneity test indicated X2 =0.46 and I2 =0%, demonstrating homogeneity.
DISCUSSION
One of the first cases of EUS-guided CPB for chronic pancreatitis was reported by Faigel et al (35). Since then, EUS-guided CPB has emerged as a promise technique and several studies have shown that this procedure has a beneficial role in the treatment of pain induced by chronic pancreatitis (16,17,21,25,29,34-37).
CPB using EUS as a tool offers multiple advantages over radiologic guidance. EUS gives real time visualization of the celiac space from the lesser curvature of the stomach. The ability to monitor the injection into the celiac space and the ganglia is a major advantage over radiologic guidance (4).
Previous studies have already showed improved pain control following CPN and CPB guided by EUS or percutaneous techniques in comparison with analgesic therapy. A meta-analysis aimed to look at the efficacy of CPB for improving pain in patients with chronic pancreatitis showed that the overall percentage who obtained pain relief with this procedure was 51%, but it was temporary, lasting from few weeks to months (34). Two meta-analysis about CPN in pancreatic cancer showed superiority of pain relief over analgesic therapy following EUS-CPB, nevertheless they concluded that the percutaneous approach remains the standard technique as robust evidence for EUS CPN was lacking (7,8). The meta-analysis of percutaneous CPN for pain showed that the pain scores in patients who underwent CPN were significantly lower compared with patients who were treated with analgesic therapy after 1–2 weeks (p =0.004) and 1 month (p =0001). After 2 months, no difference was found (20,23).
This is the first systematic review comparing EUS and percutaneous-guided CBP on treatment of abdominal pain due to chronic pancreatitis. Our analysis demonstrated that EUS-CPB is more effective in reduce pain only in 4 weeks, with no statistically significant difference in 1, 8 and 12 weeks after the procedure. Moreover, no statistically significant difference in complications following the procedures was observed.
Some limitations need to be acknowledged in this systematic review. Although the qualifications for JADAD studies adopted its selection, sample size and differences in techniques compared gave the meta-analysis a significant risk of bias, which does not allow statistically significant statements, phenomenon evidenced especially in the first 4 weeks of treatment, where the differences appear between the two procedures was evident, but with heterogeneity between the studies. The quality of the evidence that exists to answer this question is small, so it is important to advance new studies with larger populations to enhance the power of the evidence.
In conclusion, according to evidence found in this meta-analysis, no statistically significant difference was noted in pain relief and complications for EUS and percutaneous – CPB.
Acknowledgements
No specific funding was obtained for this work. The authors declare no conflict of interest.
REFERENCES
1. Michaels AJ, Draganov PV. Endoscopic ultrasonography guided celiac plexus neurolysis and celiac plexus block in the management of pain due to pancreatic cancer and chronic pancreatitis . World J Gastroenterol. 2007;13(26):3575-80. [ Links ]
2. Talukdar R, Reddy DN. Pain in chronic pancreatitis: managing beyond the pancreatic duct . World J Gastroenterol. 2013;19(38):6319-28. [ Links ] .
3. Rana MV, Candido KD, Raja O, Knezevic NN. Celiac plexus block in the management of chronic abdominal pain . Curr Pain Headache Rep. 2014;18(2):394. [ Links ]
4. Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review . Dig Dis Sci. 2009;54(11):2330-7. [ Links ]
5. Stevens T. Update on the role of endoscopic ultrasound in chronic pancreatitis . Curr Gastroenterol Rep. 2011 Apr;13(2):117-22. [ Links ]
6. Penman ID, Rösch T; EUS 2008 Working Group. EUS 2008 Working Group document: evaluation of EUS-guided celiac plexus neurolysis/block (with video) . Gastrointest Endosc. 2009 Feb;69(2 Suppl):S28-31. [ Links ]
7. Nagels W, Pease N, Bekkering G, Cools F, Dobbels P. Celiac plexus neurolysis for abdominal cancer pain: a systematic review . Pain Med. 2013 Aug;14(8):1140-63. [ Links ]
8. Zhong W, Yu Z, Zeng JX, Lin Y, Yu T, Min XH, et al. Celiac plexus block for treatment of pain associated with pancreatic cancer: a meta-analysis . Pain Pract. 2014 Jan;14(1):43-51. [ Links ]
9. Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis . Gastrointest Endosc. 1996 Dec;44(6):656-62. [ Links ]
10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration . J Clin Epidemiol. 2009 Oct;62(10):e1-34. doi: 10.1016/j.jclinepi.2009.06.006. [ Links ]
11. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1-12. [ Links ]
12. Gao L, Yang YJ, Xu HY, Zhou J, Hong H, Wang YL, et al. A randomized clinical trial of nerve block to manage end-stage pancreatic cancerous pain . Tumour Biol. 2014 Mar;35(3):2297-301. [ Links ]
13. Amr YM, Makharita MY. Comparative study between 2 protocols for management of severe pain in patients with unresectable pancreatic cancer: one-year follow-up . Clin J Pain. 2013 Sep;29(9):807-13. [ Links ]
14. Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, et al. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial . Endoscopy. 2013;45(5):362-9. [ Links ]
15. LeBlanc JK, Al-Haddad M, McHenry L, Sherman S, Juan M, McGreevy K, et al. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011 Dec;74(6):1300-7. [ Links ]
16. Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer . J Clin Oncol. 2011 Sep 10;29(26):3541-6. [ Links ]
17. Sakamoto H, Kitano M, Kamata K, Komaki T, Imai H, Chikugo T, et al. EUS-guided broad plexus neurolysis over the superior mesenteric artery using a 25-gauge needle . Am J Gastroenterol. 2010 Dec;105(12):2599-606. [ Links ]
18. Santosh D, Lakhtakia S, Gupta R, Reddy DN, Rao GV, Tandan M, et al. Clinical trial: a randomized trial comparing fluoroscopy guided percutaneous technique vs. endoscopic ultrasound guided technique of coeliac plexus block for treatment of pain in chronic pancreatitis . Aliment Pharmacol Ther. 2009 May 1;29(9):979-84. [ Links ]
19. LeBlanc JK, DeWitt J, Johnson C, Okumu W, McGreevy K, Symms M, et al. A prospective randomized trial of 1 versus 2 injections during EUS-guided celiac plexus block for chronic pancreatitis pain . Gastrointest Endosc. 2009 Apr;69(4):835-42. [ Links ]
20. Sahai AV, Lemelin V, Lam E, Paquin SC. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness . Am J Gastroenterol. 2009 Feb;104(2):326-9. [ Links ]
21. Johnson CD, Berry DP, Harris S, Pickering RM, Davis C, George S, et al. An open randomized comparison of clinical effectiveness of protocol-driven opioid analgesia, celiac plexus block or thoracoscopic splanchnicectomy for pain management in patients with pancreatic and other abdominal malignancies . Pancreatology. 2009;9(6):755-63. [ Links ]
22. Zhang CL, Zhang TJ, Guo YN, Yang LQ, He MW, Shi JZ, et al. Effect of neurolytic celiac plexus block guided by computerized tomography on pancreatic cancer pain . Dig Dis Sci. 2008 Mar;53(3):856-60. [ Links ]
23. Basinski A, Stefaniak T, Vingerhoets A, Makarewicz W, Kaska L, Stanek A, et al. Effect of NCPB and VSPL on pain and quality of life in chronic pancreatitis patients . World J Gastroenterol. 2005 Aug 28;11(32):5010-4. [ Links ]
24. Süleyman Ozyalçin N, Talu GK, Camlica H, Erdine S. Efficacy of coeliac plexus and splanchnic nerve blockades in body and tail located pancreatic cancer pain . Eur J Pain. 2004 Dec;8(6):539-45. [ Links ]
25. Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial . JAMA. 2004 Mar 3;291(9):1092-9. [ Links ]
26. Staats PS, Hekmat H, Sauter P, Lillemoe K. The effects of alcohol celiac plexus block, pain, and mood on longevity in patients with unresectable pancreatic cancer: a double-blind, randomized, placebo-controlled study . Pain Med. 2001 Mar;2(1):28-34. [ Links ]
27. Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain . Am J Gastroenterol. 1999 Apr;94(4):900-5. [ Links ]
28. Polati E, Finco G, Gottin L, Bassi C, Pederzoli P, Ischia S. Prospective randomized double-blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer . Br J Surg. 1998 Feb;85(2):199-201. [ Links ]
29. Kawamata M, Ishitani K, Ishikawa K, Sasaki H, Ota K, Omote K, et al. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain . Pain. 1996 Mar;64(3):597-602. [ Links ]
30. Ischia S, Ischia A, Polati E, Finco G. Three posterior percutaneous celiac plexus block techniques. A prospective, randomized study in 61 patients with pancreatic cancer pain . Anesthesiology. 1992 Apr;76(4):534-40. [ Links ]
31. Madsen P, Hansen E. Coeliac plexus block versus pancreaticogastrostomy for pain in chronic pancreatitis. A controlled randomized trial . Scand J Gastroenterol. 1985 Dec;20(10):1217-20. [ Links ]
32. Jain PN, Shrikhande SV, Myatra SN, Sareen R. Neurolytic celiac plexus block: a better alternative to opioid treatment in upper abdominal malignancies: an Indian experience . J Pain Palliat Care Pharmacother. 2005;19(3):15-20. [ Links ]
33. Mercadante S. Celiac plexus block versus analgesics in pancreatic cancer pain . Pain. 1993 Feb;52(2):187-92. [ Links ]
34. Polati E, Luzzani A, Schweiger V, Finco G, Ischia S. The role of neurolytic celiac plexus block in the treatment of pancreatic cancer pain . Transplant Proc. 2008 May;40(4):1200-4. [ Links ]
35. Lillemoe KD, Cameron JL, Kaufman HS, Yeo CJ, Pitt HA, Sauter PK. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial . Ann Surg. 1993 May;217(5):447-55. [ Links ]
36. Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, et al. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer . J Clin Gastroenterol. 2010 Feb;44(2):127-34. [ Links ]
37. Faigel DO, Veloso KM, Long WB, Kochman ML. Endosonography-guided celiac plexus injection for abdominal pain due to chronic pancreatitis . Am J Gastroenterol. 1996 Aug;91(8):1675. [ Links ]
38. Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience . Am J Gastroenterol. 2001 Feb;96(2):409-16. [ Links ]
39. Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct Ganglia neurolysis and block . Am J Gastroenterol. 2008 Jan;103(1):98-103. [ Links ]
Correspondence: Renata Nobre Moura
Gastrointestinal Endoscopy Unit, University of São Paulo School of Medicine Rua Dr. Enéas de Carvalho Aguiar, 255, 7 andar, CEP 05422-090, São Paulo, SP, Brazil
E-mail: nobre_renata@yahoo.com.br
Recibido: 14-07-2015
Aprobado: 22-09-2015