Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.69 no.2 Lima abr./jun. 2023 Epub 06-Jul-2023
http://dx.doi.org/10.31403/rpgo.v69i2511
Original paper
Usefulness of fetal renal length in the prediction of gestational age
1 Department of Obstetrics and Gynecology, Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela
2 Faculty of Medicine, The University of Zulia, Maracaibo, Venezuela
Objective:
To establish the usefulness of fetal kidney length measurement in the prediction of gestational age.
Design:
Prospective, longitudinal cohort study. Institution: Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela.
Methods:
Biparietal diameter, abdominal circumference, femur length, and fetal kidney length were measured during the duration of pregnancy.
Results:
Data from 215 pregnant women was selected. A total of 3,291 total evaluations were performed, with the lowest number of evaluations at 31 weeks (128), and the highest number (157) at 28 weeks. Fetal kidney length presented strong, positive and significant correlations with gestational age by date of last menstrual period and by ultrasound measurements (p < 0.001). The model of gestational age predicted by the transverse diameter of the cerebellum reached a value of the coefficient of determination of 0.682. The correlation between gestational age by date of last menstrual period and that predicted by the model reached a value of r = 0.826 (p< 0.001).
Conclusion:
Measurement of fetal kidney length is useful for predicting gestational age and together with other routine ultrasound measurements may improve the ability of current prediction models.
Key words: Fetus; Fetal development; Biometry; Kidney; Prenatal diagnosis; Ultrasonography; prenatal; Gestational age
Introduction
Accurate determination of gestational age (GA) is critical both to establish fetal health and probable delivery date and to diagnose any growth faltering. Uncertain GA is associated with increased risk of adverse perinatal outcomes including low birth weight, spontaneous preterm delivery, and mortality, regardless of maternal characteristics1.
The three basic methods for estimating GA are date of last menstrual period (LMP), clinical examination, and ultrasonography. The first two are subject to considerable error and are only useful when not available in institutions lacking ultrasound equipment2. Ultrasonography plays a key role as an integral part of obstetric practice. Ultrasound estimation of GA is derived from a model based on the combination of fetal biparietal diameter (BPD), femur length (FL) and fetal abdominal circumference (AC)3. However, as pregnancy progresses, these parameters become increasingly unreliable for predicting GA, due to the biological variations that the fetus may experience. These variations may be caused by maternal age, parity, gestational weight gain, geographic location, and specific population characteristics4,5. There are also technical factors, such as inter-observer error and different measurement techniques, which contribute to the variability of measurements as pregnancy progresses6.Several non-traditional ultrasound parameters have been evaluated to predict GA, such as trans-cerebellar diameter, clavicle length, epiphyseal ossification centers, amniotic fluid volume, echogenicity and transverse diameter of the colon and placental thickness7. Fetal renal length (FRL) is one of those alternative parameters to establish GA during the second and third trimester. This parameter can be measured from 18 weeks of gestation. It has constant growth during pregnancy and is affected by growth abnormalities. Previous studies reported that LRF is strongly related to GA8,9. However, little information is available from studies in Venezuelan and Latin American pregnant women.
The objective of this research was to establish the usefulness of fetal renal length measurement in the prediction of gestational age.
Materials and methods
A longitudinal and prospective study was performed between January 2016 and August 2022 in women with low-risk singleton pregnancies who attended the prenatal clinic of the Central Hospital of Maracaibo, Venezuela, for routine ultrasound evaluation of pregnancy. After explaining the procedure and the potential risks to the selected women, the patients signed the written informed consent. The study was approved by the Hospital Ethics Committee and was conducted in accordance with the principles of the Declaration of Helsinki.
Pregnant women between 18 and 40 years of age, with regular menstrual cycles, accurate LMP in the 6 months prior to conception and GA between 18 and 20 weeks according to LMP and who were followed up to 40 weeks were included in the study. In addition, all were required to have ultrasound assessments of craniocaudal length performed during the first trimester of pregnancy.
Women with multiple pregnancies, fetal growth restriction (IUGR), amniotic fluid volume abnormalities, chronic or pregnancy-induced hypertension, bleeding in the first or second half of pregnancy, fetal anomalies, history of smoking, illicit drug use, endocrinopathies, heart disease, kidney disease, and differences of 2 weeks or more between GA by LMP and GA established by first-trimester ultrasound evaluation were excluded. Patients were also excluded if all four measurements had not been taken at the time of evaluation and if they missed at least three(3) consecutive follow-up visits.
After appropriate questioning and physical examination, the different fetal ultrasound measurements were performed: FRL, BPD, AC and FL. All these measurements were performed in the same trans-abdominal evaluation with the pregnant women in the supine position and using a 730-Expert® ultrasound scanner (Voluson, Austria) and a 3.5 MHz curvilinear transducer. All patients in the study were evaluated every two weeks and the measurements of the parameters were done by two physicians specialized in maternal-fetal medicine with experience in fetal ultrasound, who did not participate in the final analysis of the results. For each ultrasound parameter measured, three measurements were obtained and the average value was used as the final value.
The BPD measurement was performed in cross section, locating the interhemispheric fissure, cavum septum pellucidum and the third ventricle. The value used was from the outer edge of the fetal parietal, closest to the transducer, to the inner edge of the farthest parietal. Fetal AC was measured in a cross section of the abdomen, just below the heart, at the level of the liver and with visualization of the intrahepatic portion of the umbilical vein, stomach and spine; the elliptical method was used with the most circular abdominal contour possible. The fetal FL was measured with an inclination of the transducer less than 45º, to eliminate angle distortion. This measurement was performed on the entire femoral extension, between the middle thirds of the distal epiphysis and proximal epiphysis (ossified diaphysis), excluding metaphysis and ossified nuclei.
LRF measurements were performed in the transverse scan, in a cephalocaudal direction until both kidneys were visualized, at or just below the level of the stomach. The transducer was oriented at a 90° angle to obtain the view of the longitudinal axis of each kidney on both sides of the abdominal aorta. All measurements were made from the outer edge of the upper pole to the outer edge of the lower pole, as previously described(10). Three measurements were taken and the average value in millimeters was used for statistical analysis. Cases in which it was impossible to perform the evaluation were excluded from the study.
A base was constructed with all the available data to elaborate a reference table for the FRL measurements with their corresponding GA. Correlations between the FRL values with the GA by LMP and the other ultrasound measurements were determined using Pearson's correlation. Subsequently, a linear regression analysis was used to obtain a prediction model of the GA based on the FRL values measured by ultrasound and another using the combination of all the ultrasound parameters. Finally, the difference between the GA by LMP and those predicted by each model was calculated. A value p < 0.05 was considered statistically significant.
Results
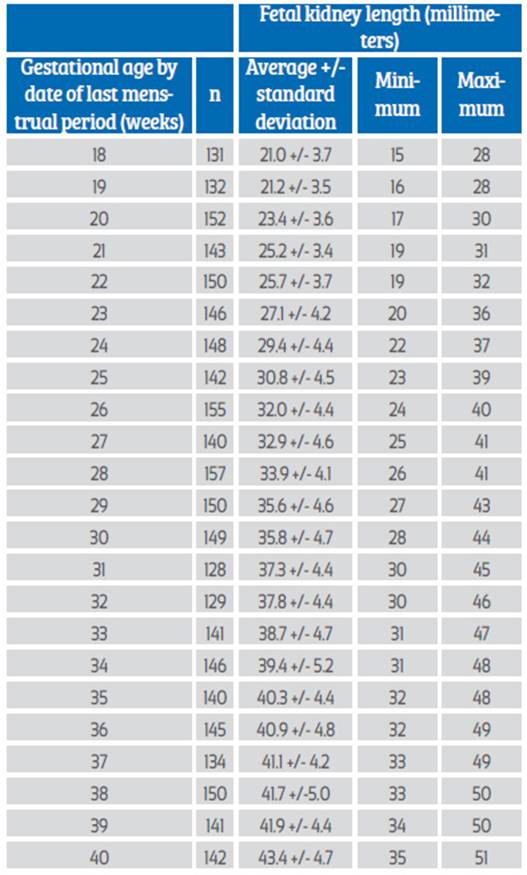
Data from 215 healthy women with singleton pregnancies followed continuously for prenatal ultrasound evaluation, with mean age of 29.3 +/- 6.8 years and 1.5 +/- 0.8 pregnancies, were selected for the final analysis. Ninety-five patients (46.3%) were primigravidae. The values for the number of evaluations and FRL between 18 and 40 weeks of gestation are shown in Table 1. A total of 3,291 total evaluations were performed, with the lowest number being 128 evaluations at 31 weeks and the highest number 157 at 28 weeks.
When analyzing the correlation between FRL with GA by LMP and the rest of the ultrasound variables evaluated, strong, positive and significant correlations were found with GA (r = 0.826; p < 0.0001), FL (r = 0.823; p < 0.001), BPD (r = 0.822; p < 0.001) and AC (r = 0.811; p < 0.0001) (Figure 2). The model of predicted GA using a linear regression model with LRF values resulted in:
GA estimated by FRL = 6.359 + (FRL * 0.670).
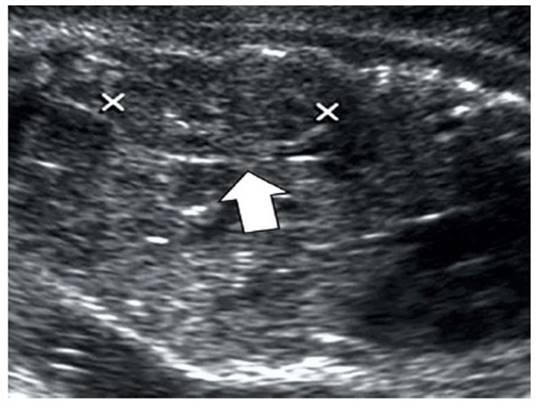
Figure 1 Prenatal ultrasound. The arrow indicates the measurement of the length of the fetal kidney.
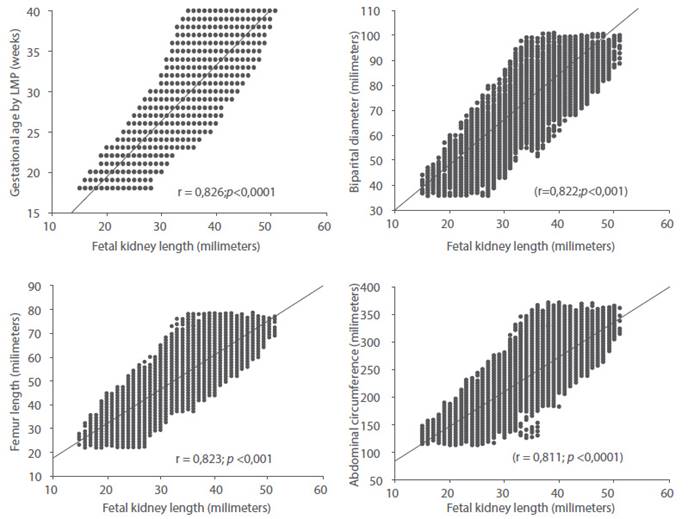
Figure 2 Correlation between fetal kidney length values and gestational age by date of last menstrual period, biparietal diameter, abdominal circumference and femur length.
The value of the coefficient of determination (r2) of the model was 0.682 (Figure 3). The average difference between GA by LMP and GA obtained by the model was +/- 2.2 weeks (16 days). When correlating with the GA by LMP, a value of r = 0.826 was observed (Figure 4). The coefficient of determination value for FRL was lower than that observed for BPD (r2 = 0.961), AC (r2 = 965) and FL (r2 = 970); when evaluated individually. However, all these values were statistically significant (p < 0.0001).
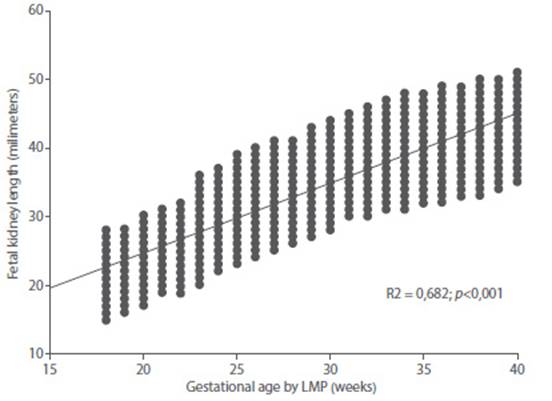
Figure 3 Graph of regression analysis between gestational age by date of last menstrual period and fetal kidney length.
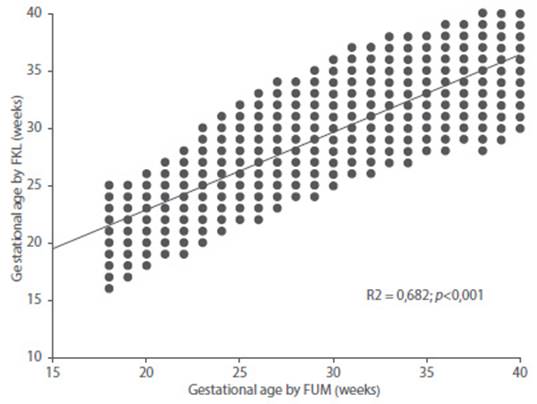
Figure 4 Correlation between gestational age by date of last menstrual period with gestational age predicted by fetal kidney length.
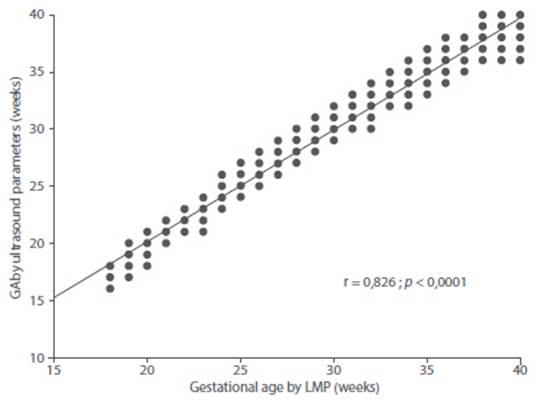
Figure 5 Correlation between gestational age by date of last menstrual period with gestational age predicted by the combination of fetal ultrasound parameters.
Combining the four ultrasound parameters studied in the resulting model was:
Estimated GA = 3.978 + ((0.086 * BPD) + (0.033 * AC) + (0.200 * FL) + (0.013 * LRF)).
The determination value of this model was 0.979. When correlating the values of GA per LMP with the results of the model, a correlation of 0.826 was found which was statistically significant (p < 0.0001). The maximum difference between the GA by LMP and that predicted by the model was +/- 0.8 weeks (5 - 6 days).
Discussion
The results of this study show that FRL is a useful measure in the prediction of GA, as it presents significant correlations with GA by LMP and the values of BPD, FL, and AC. These results are similar to previous studies demonstrating that FRL is useful in predicting GA(11). Other investigations confirmed that the measurements can be used as an additional ultrasound parameter in the routine assessment of fetal well-being and to rule out renal malformations due to size change(10).
Accurate determination of GA has marked benefits in perinatal medicine. However, when women are unsure of their LMP, it can be difficult to date the pregnancy. Therefore, it is necessary to use ultrasonography to attempt to establish accurate GA during the late second and third trimester. The advent of high-resolution equipment has improved the ability to image fetal organs(12). Efforts have been directed at finding an appropriate biometric parameter that can better predict gestational age as the pregnancy progresses to term. Such a biometric parameter should have little biological variation and be easily measured with a high degree of reproducibility and reliability.
The fetal kidney fulfills these conditions, since its growth is constant throughout pregnancy (increasing in a linear fashion, approximately 1.7 millimeters every two weeks) and is not affected by growth disturbances(13-15). The development of the fetal kidneys follows a complex but distinctive sequential pattern that begins around 7-8 weeks and continues until 35-36 weeks(11). Fetal kidneys are difficult to identify by ultrasound before 17 weeks but are visible in approximately 90% of fetuses between 17-22 weeks. During the second trimester, fetal kidneys appear as hypoechoic, oval structures in the retroperitoneum surrounding the pyelocaliceal sinus, slightly more echogenic, but lacking distinct borders. As they develop, the pyelocaliceal system becomes more noticeable. At around 27 weeks, the renal pyramids and capsule are evident(16). As pregnancy progresses, perinephric fat deposits help to accurately identify the renal borders by differentiating them from adjacent soft tissues(16,17). Around 30 weeks, perinephric fat accentuates the renal contour(18). Alterations in fetal growth predominantly affect the antero-posterior and transverse diameters of the fetal kidneys without altering their length(11, 16, 17).
The results of this study confirm that FRL is a useful measure with high predictive value that can be used as a complementary parameter to traditional measurements. Other studies have demonstrated the usefulness of FRL in predicting GA. One investigation showed that there was a strong correlation between the two variables during the third trimester of pregnancy, even in cases of intrauterine growth restriction of the fetus(15). Another study concluded that there was a positive and significant correlation between the two variables with a linear growth rate throughout pregnancy(19). A subsequent investigation indicated that FRL had a high correlation with GA and other ultrasound biometric values on an individual basis(20). Finally, one research group proposed that FRL was the most accurate single parameter for estimating GA compared to other biometric parameters, especially in cases of IUGR and some maternal conditions in which other ultrasound parameters may not be reliable for establishing GA(21).
The results of this investigation also confirmed that FRL measurement has similar predictive capabilities for GA as BPD, AC and FL individually. However, several authors have reported that its predictive ability is superior to the other ultrasound parameters in predicting GA(13, 16, 22, 23). This is an important aspect, since previous research has described that BPD loses predictive value as pregnancy progresses to term, due to the fitting - modeling of the cephalic pole and the difficulty in obtaining the standard plane of measurement(4).
The correlation between FRL and GA predicted by the combination of all ultrasound markers in this investigation is similar to what has been previously reported(13, 14, 16, 17, 22, 24). In addition, significant correlations have been shown to exist between FRL with LMP and with estimated third trimester GA by a single ultrasound measurement (25).
In the regression model, the value of FRL was r2 = 0.682 for predicting GA, which is clinically useful. In addition, the regression model using the four ultrasound parameters achieved a value of r2 = 0.979 which can improve the predictive ability of traditional ultrasound measurements. The model for estimating GA at the end of pregnancy, which included the variables LRF, BPD, FL, and AC, accurately predicted gestational age with a standard error of +/- 0.8 weeks. This model was slightly more accurate than those derived from biometric indices using BPD and FL (+/- 1.40 weeks), BPD, FL, and AC (+/- 1.35 weeks) and BPD and FL (+/- 1.43 weeks). Individually, both FRL and FL were the most accurate individual parameters for predicting GA using simple linear regression models (+/- 1.47 and 1.56 weeks, respectively). AC was the least accurate (+/- 2.0 weeks) (13). The standard error of prediction of GA obtained from a multivariate model has been shown to be lower than that obtained when using a single variable. This agrees with results of other authors who recommend that ultrasound estimation of GA should be the result of the combination of different fetal biometric measurements (5, 26, 29).
Importantly, combining the FRL value with other routine fetal biometric measurements can be useful in predicting GA in fetuses susceptible to growth faltering or when there are pathologies that preclude its measurement such as achondroplasia, phocomelia, amelia, fetal hepatosplenomegaly, cranial agenesis or anencephaly (2). In addition, it is valuable in circumstances where BPD or fetal head circumference cannot be measured.
The main strength of this study is that it is the first known investigation that confirms the usefulness of FRL in predicting GA in Latin American and Venezuelan patients during the second and third trimester of pregnancy. In addition, the sample and number of observations is one of the largest published to date. Among the limitations is that FRL measurement may be difficult to perform in fetuses with breech presentation or vertex presentation with the back oriented laterally or posteriorly. In addition, technical errors or maternal obesity may lead to poor assessments and prevent identification of the fetal kidneys, especially during the second trimester, when the fetal adrenal and renal parenchyma have homogeneous patterns. This can be corrected by modifying the transducer position and insonation angle. Accuracy may be affected by sonographer experience, equipment quality, observer bias, and population differences.
REFERENCES
1. Abonyi EO, Eze CU, Agwuna KK, Onwuzu WS. Sonographic estimation of gestational age from 20 to 40 weeks by fetal kidney lengths' measurements among pregnant women in Portharcourt, Nigeria. BMC Med Imaging. 2019;19(1):72. doi: 10.1186/s12880-019-0371-z. [ Links ]
2. Ugur MG, Mustafa A, Ozcan HC, Tepe NB, Kurt H, Akcil E, et al. Fetal kidney length as a useful adjunct parameter for better determination of gestational age. Saudi Med J. 2016;37(5):533-7. doi: 10.15537/smj.2016.5.14225. [ Links ]
3. Butt K, Lim KI. Guideline No. 388-Determination of Gestational Age by Ultrasound. J Obstet Gynaecol Can. 2019;41(10):1497-1507. doi: 10.1016/j.jogc.2019.04.010. [ Links ]
4. Ortega-Villa AM, Albert PS. Estimating onset time from longitudinal data in the presence of measurement error with application to estimating gestational age from maternal anthropometry during pregnancy. Stat Med. 2018;37(30):4743- 4757. doi: 10.1002/sim.7955. [ Links ]
5. Laurent AC, Blanc J, Grangé G. Is the stomach a main landmark on the abdominal circumference? Audit of 3 operators. J Gynecol Obstet Biol Reprod (Paris). 2016;45(5):484-9. doi: 10.1016/j.jgyn.2015.05.002. [ Links ]
6. Self A, Papageorghiou AT. Ultrasound diagnosis of the small and large fetus. Obstet Gynecol Clin North Am. 2021;48(2):339-357. doi: 10.1016/j.ogc.2021.03.003. [ Links ]
7. Silva PIP, Perez M. Prenatal ultrasound diagnosis of biometric changes in the brain of growth restricted fetuses. A systematic review of literature. Rev Bras Ginecol Obstet. 2021;43(7):545-559. doi: 10.1055/s-0041-1730290. [ Links ]
8. Awazu M. Structural and functional changes in the kidney caused by adverse fetal and neonatal environments. Mol Biol Rep. 2022;49(3):2335-2344. doi: 10.1007/s11033-021- 06967-w [ Links ]
9. Edevbie JP, Akhigbe AO. Ultrasound measurement of fetal kidney length in normal pregnancy and correlation with gestational age. Niger J Clin Pract. 2018;21(8):960-966. doi: 10.4103/njcp.njcp_373_15. [ Links ]
10. Osho ES, Ibitoye BO, Adetiloye VA, Adeyemi AB, Aderibigbe AS, Omisore AD. Ultrasonic determination of gestational age by assessment of fetal kidney size in the third trimester in southwest Nigeria. Int J Gynaecol Obstet. 2019;144(3):271- 276. doi: 10.1002/ijgo.12758. [ Links ]
11. Seilanian Toosi F, Rezaie-Delui H. Evaluation of the normal fetal kidney length and its correlation with gestational age. Acta Med Iran. 2013;51(5):303-6. [ Links ]
12. Unger H, Thriemer K, Ley B, Tinto H, Traoré M, Valea I, et al. The assessment of gestational age: a comparison of different methods from a malaria pregnancy cohort in sub-Saharan Africa. BMC Pregnancy Childbirth. 2019;19(1):12. doi: 10.1186/s12884-018-2128-z. [ Links ]
13. Konje JC, Abrams KR, Bell SC, Taylor DJ. Determination of gestational age after the 24th week of gestation from fetal kidney length measurements. Ultrasound Obstet Gynecol. 2002;19(6):592-7. doi: 10.1046/j.1469-0705.2002.00704.x. [ Links ]
14. Brennan S, Watson D, Rudd D, Schneider M, Kandasamy Y. Evaluation of fetal kidney growth using ultrasound: A systematic review. Eur J Radiol. 2017;96:55-64. doi: 10.1016/j.ejrad.2017.09.017. [ Links ]
15. Shivalingaiah N, Sowmya K, Ananya R, Kanmani TR, Marimuthu P. Fetal kidney length as a parameter for determination of gestational age in pregnancy. Int J Reprod Contracept Obstet Gynecol. 2014;3(2):424-7. [ Links ]
16. Kansaria JJ, Parukelar SV. Normogram for fetal kidney. Bombay Hosp J. 2009;51:592-7. [ Links ]
17. Cohen HL, Cooper J, Eisenberg P, Mandel FS, Gross BR, Goldman MA, et al. Normal length of fetal kidneys: sonographic study in 397 obstetric patients. AJR Am J Roentgenol. 1991;157(3):545-8. doi: 10.2214/ajr.157.3.1872242. [ Links ]
18. Yusuf N, Moslem F, Haque JA. Fetal kidney length: Can be a new parameter for determination of gestational age in 3rd trimester. TAJ: Journal of Teachers Association. 2009;20(2),147-150. doi: 10.3329/taj.v20i2.3078 [ Links ]
19. Bardhan J, Ghosh SK, Sarkar KN, Sarkar M. Fetal kidney length as a parameter for gestational age determination and its comparative evaluation with other fetal biometric indices. IAIM. 2016;3(8):36-44. [ Links ]
20. Chatterjee S, Yadav K, Prakash P, Shekhawat K. Foetal kidney length as a parameter for determination of gestational age in pregnancy by ultrasonography. IJRCOG;2016;5(6):1949-52. doi: 10.18203/2320-1770.ijrcog20161696 [ Links ]
21. Singh A, Singh G, Gupta K. Estimation of gestational age by using fetal kidney length and transcerebellar diameter in comparison with other biometric indices. Donald School J Ultrasound Obstet Gynecol 2021;15(1):4-9. [ Links ]
22. Kumar K, Lalwani R, Babu R, Aneja S, Malik A. Ultrasonographic estimation of fetal gestational age by fetal kidney length. J Anat Soc India 2013;62(1):33-36. doi: 10.1016/S0003-2778(13)80009-3. [ Links ]
23. Kaul I, Menia V, Anand AK, Gupta R. Role of fetal kidney length in estimation of gestational age. JK Science 2012;14(2):65-69. [ Links ]
24. Abbas F, Javed M, Ali H, Wazir F. Comparative study of manual and ultrasonographic measurement of fetal renal length. Gomal J Med Sci. 2012;10(1):27-31. [ Links ]
25. Joylene DD, Anjum I, Sujaya VR. A comparative study to determine the gestational age in third trimester using mean fetal kidney length versus multiple biometric parameters. J Evid Based Healthc 2015;2:4034-44 [ Links ]
26. Paoletti D, Smyth L, Westerway S, Hyett J, Mogra R, Haslett S, et al. A survey of current practice in reporting third trimester fetal biometry and Doppler in Australia and New Zealand. Australas J Ultrasound Med. 2021;24(4):225-237. doi: 10.1002/ajum.12282. [ Links ]
27. DeVore GR. How to avoid errors when computing reference interval tables and graphs using regression equations for cross-sectional studies of fetal biometry. Ultrasound Obstet Gynecol. 2022;60(2):163-166. doi: 10.1002/uog.24875. [ Links ]
28. Choi HY, Kim JH, Park JY, Jung EY, No JH, Oh KJ, et al. Simple mathematical formulae for estimation of median values of fetal biometry at each gestational age. Obstet Gynecol Sci. 2016;59(2):91-6. doi: 10.5468/ogs.2016.59.2.91. [ Links ]
29. Aggarwal N, Sharma GL. Fetal ultrasound parameters: Reference values for a local perspective. Indian J Radiol Imaging. 2020;30(2):149-155. doi: 10.4103/ijri.IJRI_287_19. [ Links ]
Statement of ethical issues
Ethical responsibilities: Protection of persons. The authors declare that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of data: The authors declare that they have followed the protocols of the Central Hospital "Dr. Urquinaona" and the University of Zulia on the publication of patient data.
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Funding: The authors certify that they have not received financial support, equipment, in working personnel or in kind from individuals, public and/or private institutions for the conduct of the study.
Received: November 11, 2022; Accepted: April 02, 2023











 texto en
texto en