Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.69 no.1 Lima ene./mar. 2023 Epub 27-Mar-2023
http://dx.doi.org/10.31403/rpgo.v69i2488
Case report
Pemphigoid gestationis. Case report
1Specialist in Obstetrics and Gynecology, Sanitas Medical Center, Coral Springs, Florida, United States.
2Doctor in Clinical Medicine, Specialist in Obstetrics and Gynecology, Obstetrics and Gynecology Service, Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela.
Pemphigoid gestationis, formerly known as herpes gestationis, is a rare, blistering, autoimmune, pregnancy-specific dermatosis. Although the etiology is not fully understood, most patients develop antibodies against 180 kDa transmembrane hemidesmosomal protein. The defining symptoms of the disease are intense itching and urticarial lesions that evolve into painful blisters. Lesions begin in the periumbilical region in 90% of cases and spread rapidly to other areas of the body, except for the head and mucous membranes. It is most frequent in the second or third trimester. In skin biopsy specimens, direct immunofluorescence staining validates the diagnosis. Topical corticosteroids can be used to treat mild symptoms, while oral corticosteroids and antihistamines should be used to treat severe cases. A case of pemphigoid gestationis is presented.
Key words: Pemphigoid gestationis; Dermatosis; Pregnancy; Pemphigus
INTRODUCTION
Pemphigoid gestationis, previously known as herpes gestationis, is a rare cutaneous, bullous, autoimmune disorder of pregnancy first described in 18721). Most cases occur in the second or third trimester, with reports of a few isolated cases during the first trimester and puerperium. The main clinical manifestations are tension skin blisters with erythematous plaques, similar to those described in urticaria, accompanied by pruritus2,3). The clinical manifestations and laboratory findings are similar to bullous pemphigoid and its exact pathogenesis is still unknown1). A case of pemphigoid gestationis is presented.
CASE REPORT
A 31-year-old female patient, gestation II, para I, with an active pregnancy of 25 weeks, consulted for presenting intense pruritus of approximately 12 days of evolution, associated with urticaria and edematous rash, initially in the peri-umbilical area, which then spread to the rest of the abdomen, thorax, and upper and lower limbs. The pregnancy had been conceived naturally and had not presented any complications up to that moment. She denied a personal history of skin disorders, autoimmune diseases, drug/food allergies or fever. She also denied any significant family history.
On physical examination, the patient exhibited extensive erythematous-edematous plaques, similar to urticaria, polymorphic, pink, with central erosions, crowned by tense vesicles, filled with clear fluid and scattered hemorrhagic crusts on abdomen, thorax and upper and lower extremities. The vesicles were predominantly located around the umbilical scar and both lower limbs. No mucosal lesions were observed. Nikolsky's and extension signs of the vesicles were negative.
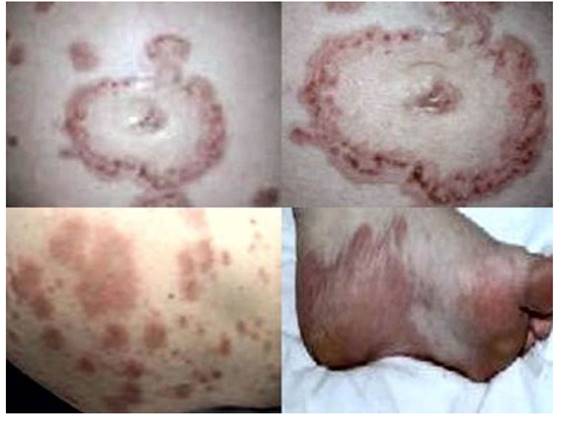
Figure 1 cutaneous lesions, erythematous plaques, vesicles, and erosions in different anatomical locations, at 25 weeks of pregnancy.
Hematology, liver and renal function, electrolytes, C-reactive protein, antistreptolysin, glycemia, urine examination, and thyroid tests were within normal limits. Obstetric ultrasound was reported normal with a male fetus in accordance with gestational age and normal amniotic fluid volume.
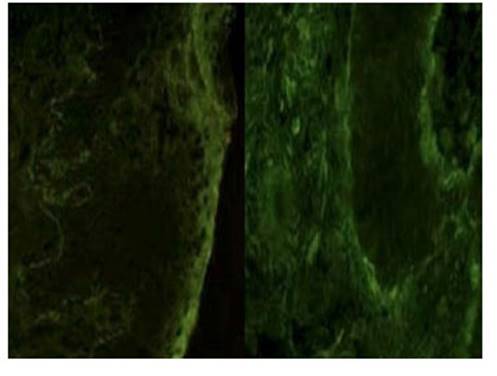
Skin biopsy was performed from the edge of the lesion on the right lower extremity. Histology showed that the dermoepidermal blister contained eosinophils with some lymphocytes and neutrophils within them. In addition, subepidermal spongiosis and perivascular lymphocytic infiltrate with granulocytes without acantholysis. Direct immunofluorescence showed positive linear staining for C3 along the basement membrane, with absence of IgA, IgG, and IgM. These results confirmed the diagnosis of pemphigoid gestationis.
In view of the findings, treatment was started with oral prednisolone (0.5 mg/kg/day), topical steroids and oral antihistamines. The clinical response was satisfactory, with a decrease in pruritus and gradual improvement of the skin lesions, which finally subsided without recurrence. The patient was discharged after 7 days with progressively decreasing doses of prednisolone and maternal-fetal surveillance.
The evolution of the pregnancy was uncomplicated, and the delivery was spontaneous resulting in a live male newborn of 3,200 grams with Apgar scores of 7 and 9 points at one minute and 5 minutes, respectively. The neonate had no skin lesions. The patient was discharged in satisfactory condition after 24 hours with no evidence of recurrence of the condition. Treatment was discontinued 15 days after the puerperium and she remained asymptomatic 6 months after discontinuation of treatment.
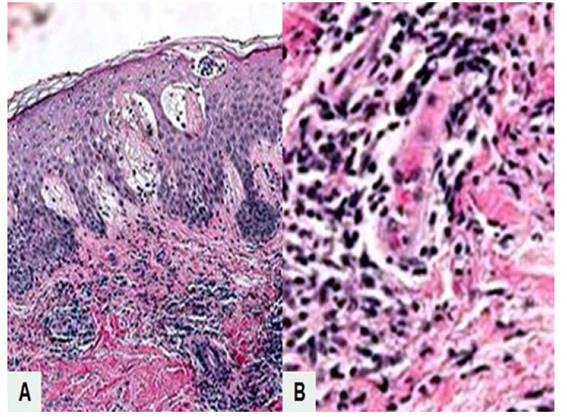
Figure 2 microscopic image of pemphigoid gestationis. a) subepidermal unfolding with fibrous material, eosinophils, lymphocytes, and neutrophils within and in the superficial dermis. hematoxylin-eosin stain, 10x). b) dermis with polymorphonuclear neutrophils and perivascular inflammatory infiltrate of the dermis (hematoxylin-eosin stain, 100x).
DISCUSSION
Pemphigoid gestationis is a rare cutaneous blistering disease with an incidence of approximately 1 case per 50,000 pregnancies4-7). The pathogenesis is autoimmune, and the triggering cause is unknown, with involvement of the dermoepidermal junction leading to blistering. The pathogenesis is similar to bullous pemphigoid. Its occurrence is associated with autoreactive IgG antibodies against BP180 protein (also known as BPAG1 or collagen XVII) and, partially, against BP230 protein, which are hemidesmosomal proteins at the dermoepidermal junction8-10). This leads to disconnection between dermis and epidermis, inflammation, and vesicle formation.
BP180 protein is expressed in skin, trophoblast, and amniotic stromal cells9). Its abnormal expression of major histocompatibility complex class II antigens on placental stromal cells leads to the presentation of BP180 protein to the maternal immune system, which recognizes it as foreign. This leads to the production of IgG antibodies that cross-react with BP180 proteins, causing inflammation and blistering. Pemphigoid gestationis is associated with HLA-DR3 and HLA-DR4 haplotypes of maternal class II antigens1).
The most common manifestations of pemphigoid gestationis are pruritus and erythematous urticarial plaques. The latter initially appear as annular plaques and pruritic papules followed by large, tense vesicles with an erythematous base. They usually appear initially in the periumbilical area and later spread to the rest of the trunk, thorax, upper and lower extremities and even hands and feet. However, there are no dermatological alterations on the face and mucous membranes11). In some patients, severe pruritus may be the only symptom, which makes diagnosis even more difficult3).
The cutaneous histologic changes of pemphigoid gestationis depend on the phase of the disease. The early phase is characterized by edema of the superficial and deep dermis with perivascular infiltrate of lymphocytes, histiocytes and eosinophils. In the bullous phase, there are subepidermal blisters filled with eosinophils and mixed perivascular infiltrate1). These findings are nonspecific and can be observed in other dermatoses. The specific diagnostic test is direct immunofluorescence of the perilesional area, characterized by linear deposits of complement C3 and less frequently IgG, along the basement membrane. C3 deposits are present in all cases, while IgG deposits appear in 25 to 50% of cases1,4). Indirect immunofluorescence and ELISA tests for circulating IgG antibodies against BP180 protein may also be useful for diagnosis and monitoring of disease activity2).
In the early phase, pemphigoid gestationis is difficult to distinguish from other dermatoses of pregnancy, including polymorphous rash, atopic rash, and intrahepatic cholestasis5). Polymorphous eruption of pregnancy shares clinical features such as onset, location, pruritus, and urticarial morphology, but usually appears late in pregnancy. Atopic rash is the most common dermatosis in pregnancy and appears in the first and second trimester. Eczematous or papular skin lesions occur mainly on the trunk and extremities. Direct immunofluorescence findings are what allow the differential diagnosis to be made between the three conditions. Intrahepatic cholestasis of pregnancy is characterized by intense pruritus. Skin lesions secondary to scratching are excoriations or pruritic nodules, usually located on the extremities. The diagnosis is confirmed by elevated bile acid levels and abnormal liver function tests12,13).
Bullous pemphigoid and pemphigoid gestationis share similar clinical, histopathological, and immunological features12). Pemphigoid gestationis shows association with HLA-DR3 and HLA-DR4, whereas bullous pemphigoid is associated with HLA-DQ3. However, the latter appears at older ages, whereas pemphigoid gestationis is exclusively related to pregnancy(3).
Pemphigoid gestationis can persist or worsen due to sudden variations in antibody concentrations and hormonal fluctuations. Therefore, it may recur in subsequent pregnancies, during menstruation or when progesterone-containing oral contraceptives are used5). There are also reports of cases associated with choriocarcinoma and hydatidiform mole14-17). Recurrences in subsequent pregnancies have been described in 33% to 50% of patients, with generally earlier onset and more severe symptomatology1,2). The most common obstetric complications are intrauterine growth restriction of the fetus and preterm delivery4,16,18).
The main goal of treatment is to reduce pruritus and prevent the formation of new blisters. In mild cases, topical corticosteroids and oral antihistamines can be used. In severe cases, oral corticosteroids such as prednisone and prednisolone are preferred as they are inactivated by the placental enzyme 11-β-hydroxysteroid dehydrogenase, producing lower fetal concentrations. Both betamethasone and dexamethasone are not metabolized and are less suitable for treatment9). Oral treatment with prednisolone starts at a dose of 0.5 mg/kg/day, which is gradually reduced according to clinical improvement(1). The duration of treatment should be individualized and depends on remission. The use of second-generation histamines is also recommended to control pruritus. In cases appearing in the puerperium there are more therapeutic options, such as intravenous immunoglobulin, azathioprine, dapsone, cyclosporine, pyridoxine, minocycline, nicotinamide, immunoadsorption, rituximab, erythromycin, cyclophosphamide, methotrexate, and plasmapheresis9,19).
It is necessary to remember that both pemphigoid gestationis and the treatment administered to pregnant women can affect fetal well-being. Most cases with pemphigoid gestationis have healthy newborns at term, delivered vaginally or abdominally for obstetric indications. Due to passive maternal-fetal transfer of antibodies, 10% of neonates may develop skin lesions. In the first trimester, prednisolone may increase the risk of malformations, especially orofacial clefts, and in the last trimester, it may cause intrauterine growth retardation, eclampsia, and preterm delivery. However, there is no increased risk of stillbirths and spontaneous abortions13,20).
In conclusion, pemphigoid gestationis is a rare autoimmune skin condition that is most common in the second and third trimester of pregnancy. It is necessary to be aware of this condition when a pregnant woman presents with pruritus and urticaria-like or vesicular skin lesions, so that treatment can be initiated in a timely manner. Treatment consists of topical or systemic corticosteroids plus antihistamines depending on the severity of the clinical picture.
REFERENCES
1. Sävervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441-9. doi: 10.2147/CCID.S128144 [ Links ]
2. Ceryn J, Siekierko A, Skibinska M, Doss N, Narbutt J, Lesiak Pemphigoid gestationis case report and review of literature. Clin Cosmet Investig Dermatol. 2021;14:665-70. doi: 10.2147/CCID.S297520 [ Links ]
3. Hallaji Z, Mortazavi H, Ashtari S, Nikoo A, Abdollahi M, Nasimi M. Pemphigoid gestationis: Clinical and histologic features of twenty-three patients. Int J Womens Dermatol. 2016;3(2):86-90. doi: 10.1016/j.ijwd.2016.11.004 [ Links ]
4. Jiao HN, Ruan YP, Liu Y, Pan M, Zhong HP. Diagnosis, fetal risk and treatment of pemphigoid gestationis in pregnancy: A case report. World J Clin Cases. 2021;9(34):10645-51. doi: 10.12998/wjcc.v9.i34.10645 [ Links ]
5. Agostinis P, Antonello RM. Pemphigoid Gestationis. N Engl J Med. 2020;383(9):e61. doi: 10.1056/NEJMicm2000922 [ Links ]
6. Guzmán YM, Hirigoity MB, Kim H, Alduncin J, Stella I, Rodriguez Cabral A. Penfigoide Gestacional. Rev Argent Dermatol. 2020;101(4):51-60. [ Links ]
7. Camero JC, Fernández E, Anuy K. Penfigoide gestacional en el tercer trimestre del embarazo. Rev Cienc Med Pinar Rio. 2020;24(5):e4445. [ Links ]
8. Cohen S, Strowd LC, Pichardo RO. Pemphigoid gestationis: a case series and review of the literature. J Dermatolog Treat. 2018;29(8):815-8. doi: 10.1080/09546634.2018.1459034 [ Links ]
9. Singla A, Shree S, Mehta S. Pregnancy with pemphigoid gestationis: A rare entity. J Clin Diagn Res. 2016;10(7):QD067. doi: 10.7860/JCDR/2016/19491.8215 [ Links ]
10. Santos-Alarcón S, Benavente-Villegas C, García-Briz I, Moneva-Léniz M, Sanchis-Sánchez C, Mateu-Puchades A. Urticarial Lesions in a Pregnant Woman. Acta Dermatovenerol Croat. 2018;26(1):71-2. [ Links ]
11. Al-Saif F, Elisa A, Al-Homidy A, Al-Ageel A, Al-Mubarak M. Retrospective analysis of pemphigoid gestationis in 32 Saudi patients Clinicopathological features and a literature review. J Reprod Immunol. 2016;116:42-5. doi: 10.1016/j.jri.2016.04.286 [ Links ]
12. Soutou B, Aractingi S. Skin disease in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29(5):732-40. doi: 10.1016/j.bpobgyn.2015.03.005 [ Links ]
13. Sävervall C, Sand FL, Thomsen SF. Dermatological diseases associated with pregnancy: Pemphigoid gestationis, polymorphic eruption of pregnancy, intrahepatic cholestasis of pregnancy, and atopic eruption of pregnancy. Dermatol Res Pract. 2015;2015:979635. doi: 10.1155/2015/979635 [ Links ]
14. Fania L, Guerriero C, Ricci F, Gagliano MF, De Simone C. Herpes gestationis and oral contraceptive: Case report and review of the literature. Dermatol Ther. 2017;30(5). doi: 10.1111/dth.12518 [ Links ]
15. Ghafoor R, Hanif IM, Ullah MA, Husseni AM. Gestational pemphigoid presenting in the second trimester of pregnancy: A rare finding. Cureus. 2022;14(5):e25531. doi: 10.7759cureus.25531 [ Links ]
16. Acosta RL, Martínez LR, Aldama A, Domínguez LM, Celías L, Mendoza G. Penfigoide gestacional asociada a complicaciones en el neonato. Rev Nac (Itauguá). 2014;6(1):53-6. [ Links ]
17. de la Cruz C, Navarrete C, Majerson D, Romero W, Vergara A, González S. Penfigoide gestacional »,» ®,® §,§ ­, ¹,¹ ²,² ³,³ ß,ß Þ,Þ þ,þ ×,× Ú,Ú ú,ú Û,Û û,û Ù,Ù ù,ù ¨,¨ Ü,Ü ü,ü Ý,Ý ý,ý ¥,¥ ÿ,ÿ ¶,¶ Herpes gestationis »,» ®,® §,§ ­, ¹,¹ ²,² ³,³ ß,ß Þ,Þ þ,þ ×,× Ú,Ú ú,ú Û,Û û,û Ù,Ù ù,ù ¨,¨ Ü,Ü ü,ü Ý,Ý ý,ý ¥,¥ ÿ,ÿ ¶,¶ : revisión a partir de un Caso Clínico. Rev Chil Obstet Ginecol. 2012;77(1):64-71. doi:10.4067/S0717-75262012000100013 [ Links ]
18. Sandoval M, Navarrete C, Araya G, González S. Penfigoide gestacional: herpes gestationis. Rev Chil Dermatol. 2012;28(3):321-3. [ Links ]
19. Kridin K, Ahn C, Huang WC, Ansari A, Sami N. Treatment update of autoimmune blistering diseases. Dermatol Clin. 2019;37(2):215-28. doi: 10.1016/j.det.2018.12.003 [ Links ]
20. Parfene CG, Bohiltea RE, Mihai BM, Grigoriu C, Margaritescu I, Chirita AD, et al. Influence of pemphigoid gestationis on pregnancy outcome: A case report and review of the literature. Exp Ther Med. 2022;23(1):23. doi: 10.3892etm.2021.10945 [ Links ]
Declaration of ethical aspects
Acknowledgement of authorship: All authors declare that we have contributed to the idea, study design, data collection, data analysis and interpretation, critical review of the intellectual content, and final approval of the manuscript we are submitting.
Ethical responsibilities: Protection of persons. We, the authors, declare that the procedures followed conformed to the ethical standards of the committee on responsible human experimentation and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of data: The authors declare that we have followed the protocols of the Hospital Central "Dr. Urquinaona" on the publication of patient data.
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Funding: The authors certify that we have not received financial support, equipment, personnel, or in-kind support from individuals, public and/or private institutions for the study.
Received: August 05, 2022; Accepted: November 11, 2022











 texto en
texto en