Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Revista Peruana de Biología
versão On-line ISSN 1727-9933
Rev. peru biol. v.17 n.2 Lima ago. 2010
Intraluminal colonization into the seminiferous tubules in mice
Colonización intraluminal en túbulos seminíferos en ratón
Luis Guzmán-Masias1,3* ; Rosmary López-Sam2,3 ; Flor Vásquez-Sotomayor2 ; Susan Pérez-Gamarra2 ; José Pino-Gaviño3 y Guillermo Llerena-Cano1
1 Grupo PRANOR. Lima, Perú.
2 Asociación PRESSERVARIS. Lima, Perú
3 Laboratorio de Reproducción y Biología del Desarrollo. Facultad de Ciencias Biológicas. Universidad Nacional Mayor de San Marcos. Lima, Peru.
Abstract
Using the primordial germ cells transplant technique, we could be able preserve and multiply pluripotent cells in the receptor for a long period of time. In this work, We aim to evaluate intraluminal colonization of a cellular gonocyte suspension from 14.5 dpc fetus. Cellular suspension with PGC's were isolated from fetus male mice by two enzymatic digestion steps, and cellular suspensions were transplanted into the rete testis of the receptor animals that were previously injected with Busulfan to decrease their own spermatogenesis. In this research the intraluminal colonization was identified in 13.27%, demonstrating that transplantation of a cellular suspension from gonocytes of fetus of 14.5 dpc containing PGCs can colonize the seminiferous tubules and support the spermatogenesis.
Keywords: primordial germ cells (PGCs), transplant germ cells, spermatogenesis, Busulfan, recovery, mouse.
Resumen
Con la técnica de trasplante de células germinales primordiales es posible preservar y multiplicar células pluripotentes en el receptor durante un largo periodo de tiempo. Nuestro objetivo fue evaluar la colonización intraluminal de una suspensión celular de gonocitos de 14,5 dpc obtenidos de fetos. La suspensión celular de CGP fueron aislados de fetos de ratones machos mediante dos pasos de digestión enzimática; luego, las suspensiones celulares fueron trasplantadas en la rete testis de los animales receptores los cuales fueron tratados previamente con Busulfan para disminuir su propia espermatogénesis. En esta investigación se comprobó la colonización intraluminal en 13,27%, lo que demuestra que el trasplante de una suspensión celular de gonocitos del feto de 14,5 dpc que contiene CGP pueden colonizar los túbulos seminíferos y además promover su propia espermatogénesis.
Palabras clave: Células Germinales Primordiales, transplante de células germinales, espermatogénesis, Busulfan, ratón.
Introduction
Primordial Germ Cells (PGCs) are the progenitors of germ cells line, and give rise to either oocyte or sperm cells with the potential to create complete individual organisms after fertilization (Wylie 1999). PGC's colonize genital crest between 10.5 to 11.5 days post copula (dpc) and enter in a premeiotic state at 12.5 dpc in mice. In male genital crests, the meiosis is arrested at G0/G1, leaving the spermatogoniums for future reactivation after birth. In mice fetus of 11.5 dpc, only Sertoli cells could be isolated from genital crests (Kimura 1999).
Basically, germ cell transplants consist in gathering germ cells from a donor and injecting them into the seminiferous tubules of a receptor; PGC's colonize the basal membrane of receptor´s seminiferous tubules in search of a nich where they can develop into stem cells. However, Jiang (1995) reported that in rats, PGC's colonized the seminiferous tubules atypically, which is denominated intraluminal spermatogenesis.
Transplant of germ cells between males of the same species has been demonstrated successfully in monkeys (Nagano et al. 2001) and pigs (Honaramooz et al. 2002), where a restart of the differentiation was observed in a period of four weeks. Testicular germ cells present a great regenerative capacity, as it demonstrated transplanting of 200 germ cells that were able to recover the fertility in infertile mice (Kanatsu et al. 2002). Therefore, after the transplant, the new generation of normal sperm cells has a potential application in reproductive medicine in animals and humans.
The colonization process can be divided into three continuous phases. The first phase is during the first week, where the transplanted cells have a random distribution through the seminiferous tubules and a few of them colonize the basal membrane. During the second phase, germ cells divide in the basal membrane (mitosis) and form a cellular monolayer. The last phase begins near the first month after the transplant and the transplanted germ cells establish a new cellular network line restarting their spermatogenesis (Nagano et al. 1999). However, in 1995 Jian & Short reported that germ cells isolated from rat fetus 15 dpc had colonized and differentiated into irregular segments of seminiferous epithelium in the lumen of seminiferous tubules of adult rats.
In this study, cells from mice 14.5 dpc genital ridges were isolated and transplanted to adult mice recipients previously treated with Busulfán, to probe the formation of minitubules in the receptors.
Material and methods
Recipient mouse preparation.- Four week-old male BALB C mice were used as recipients. They were maintained with photoperiod of 14:10 h (light:dark), and were fed with balanced food (Purina-Peru) and water ad libitum.
Busulfan reduces the spermatogenesis drastically (Guzmán et al. 2005, Ogawa et al. 1999) so in order to simulate mice sterility, four weeks before cell transplant, eight mice received 40 mg/kg (body weight) of Busulfán (GLAXO) intraperitoneally dissolved in dimethyl sulfoxide - DMSO (BAKER) and sterile distilled water (1:1) to obtain a final concentration of 4 mg/mL and then kept between 35 and 40 ºC.
Donor cells (PGC`s) preparation.- PGC's were isolated from male genital ridges of 14,5 dpc fetal mice after two enzymatic digestion steps. The suspension was incubated at 37 ºC with 10 volumes of Hanks Balanced Saline Solution without calcium and magnesium (HBSS) (Gibco, BRL), 1 mg/mL collagenase type IV (Gibco, BRL) and was kept under constant agitation for 15 minutes. Then, it was washed 4 times with 10 volumes of HBSS, followed by incubation at 37 ºC with HBSS containing 1mM EDTA and 0.25% of trypsin for 5 minutes. To stop trypsin action, 10 to 20% fetal bovine serum was added. After centrifugation (100 g for 10 minutes), pellets were resuspended in HBSS medium and maintained at 4 ºC until they were injected in the testes of recipient mice. The suspension was colored with Tripan Blue stain to evaluate the correct entry of PGC's into the seminiferous tubules. The cellular concentration was 300 x 106 cells/mL.
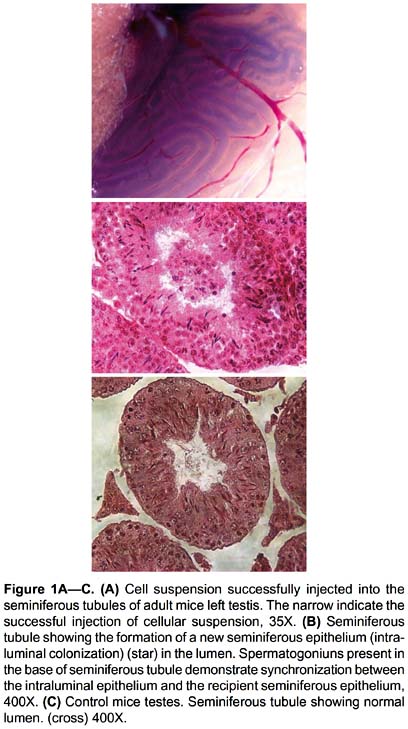
Microinjection in rete testis.- The cellular suspension was injected directly into the rete testis of the left testicle (Fig. 1A), while the right testis was used as control.
PGC´s were injected using acute glass pipettes as described by Ogawa et al. (1997), but instead of using a micromanipulator the procedure was done manually. The pressure of the pipette was not increased abruptly since an internal rupture of the rete testis limits may occur. The volume injected in each testis varied between 0.1 and 0.3 mL.
Analysis of recipient mice.- After 54 days after injection, mice were euthanized. Testes were removed and fixed in Bouin`s solution, embedded in paraffin an stained with Hematoxilina and Eosin Y. The percentage of little tubules formed into seminiferous tubules of the recipient mouse was at random in 200 seminiferous tubules by mouse.
Results
PGC's in cell suspension were isolated after two enzymatic digestion steps and colored with Tripan Blue dye, presenting good morphologic characteristics under the microscope such as roundness, smooth edges, no vacuolization and maintenance of the membrane integrity. These characteristics were present in 60% of the cell suspension after 4 hours of isolation.
Cell suspension was successfully injected into the seminiferous tubules of adult mice, as observed in Figure 1A. Fifty-four days after injections, the histological evaluation showed the presence of an intraluminal colonization in 6 of 8 injected testes (75%); in these only 13.27% of the seminiferous tubules showed the formation of a new seminiferous epithelium (named intraluminal epithelium or minitubules) in the lumen of the seminiferous tubules and was visualized as truncated segments (Fig. 1B).
Intraluminal epithelium showed synchronization with recipient seminiferous epithelium, which can be clearly observed in Figure 1B, where spermatogoniuns are present in the base of seminiferous tubule. Minitubules formed by the transplant of the PGCs have similar organization inside the seminiferous epithelium of recipient mice. The testes that did not receive the transplant (right testis) did not present intraluminal epithelium (Fig. 1C).
Discussion
PGC's are oocytes and spermatozoa precursors and constitute the only cellular lineage able to transmit the genome to the following generation; therefore, PGC's are essential for species survival. Male germ cells, like other stem cells, have two primordial characteristics: the capability to self-renew, and the potential to divide and generate cells capable of differentiation (Ogawa 1997).
The present article shows that isolated cells from mice genital ridges can give rise to the formation of an atypical intraluminal epithelium inside the seminiferous tubules of recipient mice. This new intraluminal epithelium is morphologically different from the one that is already present in the host and synchronization between the intraluminal epithelium and the recipient seminiferous epithelium like present in rats as reviewed by Jian and Short (1995).
Under normal physiological conditions, the primary spermatocyte (in preleptotene) migrates through the junctions of the Sertoli cells from the basal membrane to the tubules intraluminal compartment. Two meiotic divisions and the spermiogenesis normally occur in the adluminal microenvironment (Jiang et al. 1998); these same processes have been observed taking place in the recipient mice, during the post-transplant of PGC`s. The normal spermatogenesis of the transplanted PGC`s indicates the success of the procedure and also shows the conversion of these cells to spermatogoniums due to the interaction with Sertoli cells from the donor or from the recipient and with the microenvironment.
Only 13.27% of the seminiferous tubules presented minitubules; these formations were not observed in the control testes, which were not injected with the isolated cellular suspension. Thus, formation of this new intraluminal epithelium was not caused by the previous treatment with Busulfan or by any step of the procedure employed (Jiang et al. 1998). However, the basal membrane of seminiferous tubules was not evaluated (Nagano, et al. 1999) this result would probable increase the colonization percentages found in this research.
PGC's migration to the basal compartment of the seminiferous tubules, where they can divide and differentiate, is due to the intercellular interactions in search of microenvironmental stem cells (Sprandling et al. 2001). This explains the recolonization of the seminiferous tubules by PGC's in experiments where W/W- mice were used and the colonization percentages obtained where high. However, under the conditions of the experiment described in this article, Busulfan does not eliminate receptor's germ cells completely and the presence of the original germinal lineage limits the basal colonization of the new PGC's; this could explains the low colonization percentages obtained. The formation of intraluminal epithelium could be due to other cells (testicular somatic precursor cells) present in the cell suspension used, which when co-transplanted could form minitubules in the seminiferous tubules capable of supporting the spermatogenesis entire process.
Therefore, germ cell and PGC transplants appear to be reliable techniques for the study of spermatogenesis, transgenic mice (Suveera et al. 2008) and have a potential application in Assisted Reproductive Techniques (ART) in humans (for the recovery of fertilizing capability of children after treatment with chemotherapy (Nagano et al. 2002, Mitchell et al. 2009) and wild mammals in extinction threat.
Acknowledgements
Laboratory of Genetic and Human Assisted Reproduction - PRANOR Group and Laboratorio de Reproducción y Biología del Desarrollo for supporting this research.
Literature cited
Guzmán L., R. López, G. Llerena, et al. 2005. Male germinal epithelium recovery in mice treated with only dose of Busulfan. Rev. peru. biol. 12: 141 - 144.
Honaramooz A., S.Megee & I. Dobrinski. 2002. Germ cell transplantation in pig. Biology of Reproduction. 66: 21 - 28.
Jiang F.X. & R.V. Short. 1995. Male germ cell transplantion in rats: apparent synchronization of spermatogenesis between host and seminiferous epithelia. International Journal of Andrology. 18: 326 - 330.
Jiang F.X. & R.V. Short. 1998 Different fate of primordial germ cells and gonocytes following transplantation. Acta Pathologica, Microbiologica et Immunologica Scandinavica. 106: 58-63.
Kanatsu M., A. Ogura, M. Ikegawa, et al. 2002. Adenovirus-mediated gene delivery and in-vitro microinsemination produce offspring from infertility male mice. Proceedings of the National Academy of Sciences. 99: 1383 - 1388.
Kimura T., K. Yomogida, N. Iwai, et al. 1999. Molecular cloning and genomic organization of mouse homologue of Drosophila germ cell and its expression in germ lineage cells. Biochemical and Biophysical Research Communications 262: 223-230.
Mitchell R.T., P.T. Saunders, R.M. Sharpe, et al. 2009. Male Fertility and strategies for Fertility Preservation following Childhood Cancer Treatment. Endocrinology Development. 15: 101-134.
Nagano M., M. Avarbock & R. Brinster. 1999. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biology of Reproduction. 60: 1429-1436.
Nagano M., J. McCarrey & R. Brinster. 2001. Primate spermatogonial stem cells colonize mouse testes. Biology of Reproduction. 64: 1409 - 1416.
Nagano M., P. Patrizio & R. Brinster. 2002. Long-term survival of human spermatogonial stem cells in mouse testes. Fertility and Sterility. 78: 1225-1233.
Ogawa T., J. Aréchaga, M. Avarbock & R. Brinster. 1997. Transplantation of testis germinal cells into mouse seminiferous tubules. The International Journal of Developmental Biology. 41: 111 - 122.
Ogawa T., I. Dobrinski, M. Avarbock & R. Brinster. 1999 Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biology of Reproduction. 60: 515 - 521.
Sprandling A., D. Drummond-Barbosa & T. Kai. 2001. Stem cells find their niche. Nature. 414: 98 - 104.
Suveera D. & Subeer, SM. 2008. Trangenesis via permanent integration of genes in repopulating spermatogonial cells in vivo. Nature Methods. 5: 601-603.
Wylie C. 1999. Germ Cell. Cell. 96: 165-174.
* Corresponding author:
Luis Guzmán-Masias, Apartado 11-0050, Lima 11. Lima, Perú.
Email Luis Guzmán-Masias: lguzmanm@gmail.com
Presentado: 19/05/2010
Aceptado: 25/08/2010
Publicado online: 14/12/2010













