INTRODUCTION
Dengue is a viral disease caused by the bite of an infected mosquito (Aedes aegypti) that has been a major public health challenge worldwide over the past decades. Dengue has shown an increasing burden of disease caused by factors such as urbanization, warmer climates, and increased human mobility. In recent years, dengue has affected more than 104 million people and caused more than 40 deaths, with Latin America being one of the regions with the highest incidence rates 1. In this region, dengue infection is mainly concentrated in countries such as Brazil or Mexico 2 and has shown a resurgence and rise in the last 50 years due to deficient public policies, scarce resources, and climate change (3, which lead to more breeding sites for the Aedes aegypti mosquito. In Peru, the cumulative cases of dengue fever in 2023 reached 271,911, which became the worst outbreak we have had to date. However, if we compare trends, up to the epidemiological week 22, 135,520 cases were reported in 2023 (of which 133186 cases were confirmed), and 247,243 cases and 233 deaths have been seen in 2024, representing an increase of 82.4% from the previous year (Figure 1), mainly affecting the regions of Lima, Piura, La Libertad, and Ica 4.
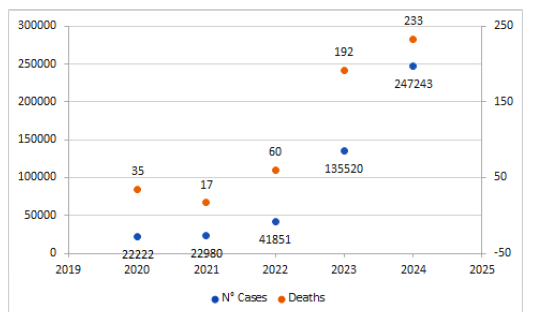
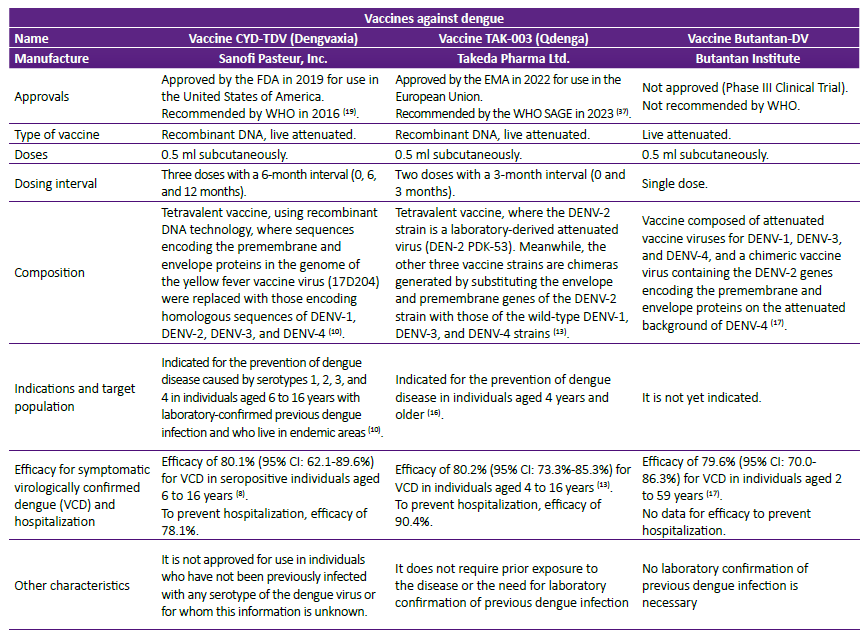
Figure 1. Number of cases and deaths in Peru between 2020 and 2024 (up to epidemiological week N° 22). Source: Dengue Situational Room Ministry of Health (MINSA) from 2024 4.
Peru, a dengue endemic country, presents the 4 serotypes of the dengue virus (DENV-1, DENV-2, DENV-3 and DENV-4) the most predominant being DENV-1 and DENV-2 5. Given the great impact of this disease, public policies have focused on implementing various strategies to stop the spread of the disease 6, such as the elimination of vectors and breeding sites, community involvement and the use of repellents and more recently, the application of vaccines. For this reason, the present article summarizes the available evidence for the different dengue vaccines that have reached the phase III of clinical trials, and it shows the current panorama on dengue vaccines in Latin America, with a special focus in Peru.
DEVELOPMENT OF THE THEME
We conducted a review of the available dengue vaccines that have progressed to phase III of clinical development, examining their efficacy and safety in preventing dengue. Additionally, we meticulously analyzed the current status of vaccination in Latin America and provided our perspective regarding the dengue vaccine implementation in Peru.
DENGUE VACCINES
To date, we have efficacy clinical trial data (Phase III) for three dengue vaccines: CYD-TDV (Dengvaxia®), TAK-003 (Qdenga®), and Butantan-DV (Table 1).
CYD-TDV (DENGVAXIA®)
It is an attenuated chimeric vaccine derived from yellow fever virus. This vaccine is administered in three doses at months 0, 6, and 12 7. Efficacy results were evaluated in clinical trials CYD15 (population aged 9-16 years in South America) and CYD14 (population aged 2-14 years in Asia). For the prevention of symptomatic virologically confirmed dengue (VCD), the combined efficacy of both clinical trials was 64.2% (95% CI: 59.6-68.4%) in people aged 6 to 16 years after a follow-up period of 25 months after inoculation of the first dose regardless of their initial serological status for dengue 8. Regarding prevention of hospitalizations, the pooled efficacy was 78.1% (68.3; 84.9) and for preventing severe disease, the efficacy was as high as 88.5% (72.0; 95.3) 8. Efficacy also varies according to dengue serotype, being higher for DENV-4 (89 %; 95 % CI 80-94 %) and lower for DENV-1 and DENV-2 serotypes (67 % for each; 95 % CI 46-80 %) 9. However, in those participants of the same age but with a positive serological status at the beginning of the study, the combined efficacy for the prevention of VCD was higher, reaching a percentage of 80.1% (Table 1) at 12-month follow-up after completing the three-dose series 8. Of note, a higher hazard ratio (HR: 6.25; 95% CI: 0.81-48.32) for severe dengue/hospitalization was reported in participants with a negative serological status at the beginning of the study, while for participants with a positive serological status the HR was lower (HR: 0.18; 95% CI: 0.09-0.37) 8.
That is why in 2019, the Food and Drug Administration (FDA) cleared Dengvaxia for the prevention of dengue (serotypes DENV-1, DENV-2, DENV-3, and DENV-4) in people aged 6 to 16 years infected with previous laboratory-confirmed dengue and living in endemic areas 10. Regarding the safety profile in this population, fever, erythema at the injection site, myalgia and headache were the most common adverse effects. Allergic and anaphylactic reactions were very rare. Adverse effects were generally short-lived and mild to moderate in severity and occurred less frequently after the second and third doses 8.
TAK-003 (QDENGA®)
It is a lived-attenuated tetravalent dengue vaccine that was developed using recombinant DNA technology based on a DENV-2 back bone 11. This vaccine is administered in two doses, with three months of separation between doses 12. The efficacy results of TAK-003 were evaluated in the DEN-301 clinical trial (population 4 to 16 years of age in South America and the Asia Pacific region). For the prevention of VCD fever due to any serotype, the efficacy was 80.2%, after a follow-up of 12 months after the second dose 12. Additionally, the efficacy for the prevention of hospitalization due to VCD fever and dengue hemorrhagic fever was 90.4% and 85.9% respectively (followup between 30 days and 18 months after administration of the second dose) 13. It is important to mention that at 54 monthfollow-up, the efficacy for preventing VCD decreased to 61.2%; however, the efficacy for hospitalization prevention remained high (84.1%) 14. In addition, when the longterm efficacy for the different serotypes were analyzed (4.5 years) in seropositive individuals, the vaccine showed good efficacy for preventing VCD and hospitalizations against all four serotypes, with the greatest efficacy reported for DENV-2, in which the reduction of VCD and hospitalizations was of 80.4% and 90.5% respectively 14.
On the other hand, in seronegative individuals, the vaccine offered good efficacy for VCD and hospitalizations against DENV1 and DENV-2; however, the vaccine was not protective against DENV-3 and showed inconclusive results for DENV-4 13. In this trial, vaccinated seronegative participants did not develop unexpected symptoms mediated by antibody-dependent enhancement; therefore, the administration is considered safe in seronegative and seropositive individuals. Although there are still no clinical efficacy results for individuals over 17 years of age, the efficacy can be inferred from immunogenicity data 12,15. The DEN-304 trial (USA) evaluated the immunogenicity of Qdenga in adults 18 to 60 years of age. By comparing the results with those found in the pediatric population, protection against dengue disease in adults (>17 years) is expected 12. Regarding safety results, in participants aged 4 to 60 years the most frequent adverse events were pain or erythema at the injection site, headache and myalgia. In the pediatric population (4 to 17 years), the most frequent adverse events, compared to adults, were fever, upper respiratory tract infection and nasopharyngitis 12. The severity of these events was mild to moderate, short in duration, and less frequent after the second dose. Therefore, in 2022, the European Medicines Agency (EMA) approved the use of Qdenga for the prevention of dengue in the European Union for individuals aged four and older. This vaccine may be administered concomitantly with hepatitis A and Yellow fever vaccines 16.
BUTANTAN-DV
The results of the DEN-03-IB clinical trial corresponding to single-dose administration of the Butantan-DV attenuated vaccine (Butantan Institute) have been published in 2024 and are very promising 17. The population in the study was composed of people aged 2 to 59 years, located in 5 geographic regions of Brazil, and the overall efficacy of the vaccine was 79.6% for the prevention of dengue VCD at 2 years of follow-up. Regarding the initial dengue serological status, the efficacy was 73.6% among participants with negative serology and 89.2% with positive serology. Likewise, according to the age group, the efficacy was 80.1% among participants aged 2 to 6 years, 77.8% among those aged 7 to 17 years and 90.0% among those aged 18 to 59 years. In addition, efficacy is different according to serotype, being 89.5% (95% CI, 78.7 to 95.0) against DENV-1, but decreasing to 69.6% (95% CI, 50.8 to 81.5) against DENV-2; no efficacy has been identified for the other serotypes 17. No data was provided about hospitalization due to the low incidence of severe dengue in this study.
The most common adverse events in the vaccine group were pain at the administration site, headache, fatigue, and rash. Most adverse events reported were mild to moderate in severity. In total, 28 serious adverse events related to the vaccine or placebo were reported (20 in the vaccine group and 8 in the control group) and although there were 16 deaths during the follow-up period (9 in the vaccine group and 7 in the control group), the researchers considered that they were not caused by dengue, nor were they related to the administration of the vaccine or placebo 17.
CURRENT STATUS OF THE VACCINATION PROGRAMS IN PERU AND REST OF LATIN AMERICA
In Latin America, the CYD-TDV (Dengvaxia) vaccine was the first vaccine that became commercially available. It was approved by many countries in this region that included El Salvador, Costa Rica, Guatemala, Mexico, Brazil, Argentina, Paraguay, Colombia, and Peru 18. In 2016, the CYD-TDV vaccine was recommended by the World Health Organization (WHO) for persons aged 9 to 45 years with confirmed previous dengue infection who live in endemic areas 19. In 2018, The WHO updated its recommendations regarding the use of Dengvaxia in 2018, based on long-term safety data, which showed that seronegative vaccine recipients had an excess risk of severe dengue compa-red to unvaccinated seronegative individuals and recommended serological testing for past dengue infection and administering the vaccine only to those who have been previously infected 19. This requirement has hampered the implementation of the CYD-TDV vaccine in many countries including Peru, given the limited availability of serologic testing in rural areas. Despite its approval, this vaccine is not currently marketed in Peru 20. It is important to mention that the FDA granted approval to CYD-TDV in 2019 for children and adolescents 6-16 years old who lived in endemic areas 10. This vaccine is currently available in Puerto Rico and is included in its pediatric national immunization schedule for children aged 9-16 years having serologic evidence of previous infection 21.
In terms of TAK-003 (Qdenga), this vaccine has received prequalification by WHO in May 2024. TAK-003 is now recommended by this organization for people aged 6 to 16 years in settings with high disease burden and transmission intensity 22. With this prequalification, the Pan American Health Organization (PAHO) and other United Nations agencies are now able to purchase this vaccine (23. On the other hand, the Food and Drug Administration (FDA) has not granted approval for TAK-003 as of June 2024. At this point, Takeda pharmaceutical has decided to withdraw its application following discussions with the FDA on aspects of data collection, which could not be addressed within the current U.S. Biologics License Application (BLS) review cycle 24.
In Latin America, Brazil became the first country to approve this vaccine in April 2023 by The National Health Surveillance Agency (ANVISA) for individuals aged 4 to 60 years old in a two-dose regimen with a 3-month interval 25. In December 2023, the implementation of a public vaccination program was initiated in this country 26. To date, the Ministry of Health of Brazil has purchased 6.5 million vaccine doses for 2024. The last batch, consisting of 1 million doses, was acquired in May 2024 27. Since early-February of 2024, the government has started a massive dengue vaccination in children between 10 to 14 years old prioritizing the locations with more reported cases such as the Federal District. Brazil, being one of the countries more affected with dengue disease, reported the circulation of the four serotypes and 2,966,339 cases in the first 12 epidemiological weeks of 2024, representing an increase of 227% compared to the same period in 2023 and an increase of 284% compared to the average of the last 5 years in this country 28. Due to the persistence of this serious issue, Brazil has also secured the acquisition of 9 million vaccines by 2025 29. On the other hand, Argentina has also approved TAK-003 in April 2023 30, which is available for sale in the private sector nationwide 31. The adoption of this preventive measure has occurred in response to the emergence of locally acquired cases across different jurisdictions, with 134,202 cases reported in the first eleven epidemiologic weeks of 2024 28,32.
Of note, the Argentinean Minister of Health has mentioned that a public vaccination campaign with TAK-003 will start in endemic regions in August 2024, but the vaccine will not be integrated into the national vaccination schedule yet 33. In other countries such as Colombia, the TAK-003 vaccine was approved in November 2023 34) but not implemented despite having 69,837 cases between the epidemiologic week 1 and 11 of 2024, representing an increase of 262% compared to the average of the last 5 years for the same period 28. In the case of Paraguay, the TAK-003 vaccine is still in process of approval and is under consideration for purchase in 2024 35.
Peru, one of the countries mostly affected by this disease in 2024, has designed a national disease control strategy that included epidemiological surveillance, timely diagnosis, healthcare workers training, control of the risk of infestation by Aedes aegypti, and educational campaigns in high-risk communities. Surprisingly, vaccination has not been considered as part of this plan 36 despite the approval of the TAK-003 vaccine by the EMA and the recommendation for its use given by the WHO Strategic Advisory Group of Experts (SAGE) on Immunization in October 2023 37. As of June 2024, the TAK-003 vaccine has not been approved in this country, however, the executive director of the Peruvian National Center for Epidemiology, Prevention and Disease Control has stated that the request for the acquisition of the TAK-003 vaccine has already been submitted to PAHO and the purchase will be done through the Revolving Fund in 2024 38. It is important to mention that unlike CYD-TDV, serologic confirmation of previous dengue infection is not required with TAK-003, which makes it suitable for implementation in countries that lack laboratory services in rural areas such as Peru. In terms of efficacy against the different serotypes, TAK-003 has demonstrated high efficacy against DENV-1 and DENV-2, which are the serotypes that predominate in Peru 5.
Regarding the Butantan-DV vaccine, it has not been approved yet; however, the researchers from the Butantan Institute plan to submit a report to ANVISA, Brazil’s health surveillance agency, in the second half of 2024 in order to apply for registration of the vaccine. They expect to have a definitive approval in 2025 39.
Although the vaccines are shown as promising tools to limit the disease burden and hospitalization rates from Dengue, it is unlikely to have an immediate impact to control current outbreaks; however, it can serve as an important measure to prevent epidemics in the medium- and long-term future. In this regard, SAGE has recommended that the vaccine should be introduced about 1-2 years prior to the age-specific peak incidence of dengue-related hospitalizations 37.
CONCLUSIONS
Although vector control is considered the main measure for dengue prevention, it has achieved only limited success in Latin America. There are many factors that have contributed to the spread of dengue in this region such as urbanization without proper planning and adequate sanitary conditions, which could create adequate environments for the proliferation of the Aedes aegypti mosquito. Climate change also plays an important role in the appearance of arboviral diseases, especially with the occurrence of “el Niño”, a climate phenomenon associated with high temperatures and storms, which is a perfect scenario for the reproduction of mosquitoes. All of which makes it necessary to adopt new control measures for the prevention of dengue that could complement the traditional approach of vector control. We believe that incorporating a dengue vaccine in Peru could serve as an important tool to limit the disease burden and decrease hospitalizations in the medium- and long-term future. We hope that this report can help to recognize vaccination not as a definitive solution to the dengue epidemic, but as a fundamental tool in the fight against this disease in highly endemic regions. It is well known that the incorporation of a vaccine against dengue in Peru represents an important public health challenge, since it requires meticulous planning and coordination of resources to guarantee broad vaccination coverage, especially in the most affected regions. However, it is imperative that public authorities promote dengue vaccination programs along with other preventive measures to reduce the incidence and severity of future dengue outbreaks.