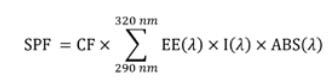Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química del Perú
versión impresa ISSN 1810-634X
Rev. Soc. Quím. Perú vol.83 no.3 Lima jul./set. 2017
TRABAJO ORIGINALES
Comparison of the Photoprotective Effects of Sunscreens Using Spectrophotometric Measurements or the Survivability of Yeast Cells Exposed to UV Radiation
Comparación de los efectos fotoprotectores de lociones bloqueadoras usando mediciones espectrofotométricas o la sobrevivencia de células de levaduras expuestas a Radiación UV
Luis Gabriel Gutiérrez Mesíasa, Anthony Mijail Romero Qwisgaarda, Giuliana Paola Chávez Untiverosa, Luciano Augusto Palomino Kobayashia, Luis Enrique Moromisato Shimabukurob, Ana Akemi Kitazono Sugaharac*
a Department of Biology – School of Sciences - Universidad Nacional Agraria La Molina
b Skinclean Laboratory, (Calle B Mz. D Lote 51 – Independencia – Lima 28 – Perú)
c* Correspondence author, Department of Chemistry – School of Sciences – Universidad Nacional Agraria La Molina, Av. La Molina S/N – La Molina – Lima 12 – Perú, Tel. +51-13-6147800 ext. 305, Email: anakitazono@lamolina.edu.pe
ABSTRACT
This work focuses on simple methods that allow comparison of the photoprotective effects of sunscreens. One such method described by Mansur and colleagues relies on the spectrophotometric measurements of the organic filters extracted with ethanol. The extracts are subjected to absorbance measurements in the of 290 to 320 nm range, with 5 nm intervals. The sunscreen sun protection factor (SPF) is estimated with an equation that relates each absorbance value with their respective erythemal effect, at the wavelength used for each measurement. In the current work, three commercial sunscreens were assayed using this method, which produced SPF values that were markedly lower than those declared by the manufacturers. These results prompted a more thorough analysis, which concluded that the Mansur method is not suitable for assaying sunscreens with SPFs above 15. The analysis included a survey of the data previously reported by several authors that had used the same method. On the other hand, this report also includes the optimization of a yeast serial dilution assay that allows reliable comparison of the photoprotection levels conferred by sunscreens. Importantly, this yeast assay could be applied to compare the photoprotective effects of products with a wide range of SPFs, including sunscreen lotions, filter suspensions or solutions, natural product extracts, etc.
Key words: Sun protection factor, SPF, UV radiation, Mansur equation
RESUMEN
Este trabajo reporta la optimización de ensayos simples para comparar los efectos fotoprotectores de bloqueadores solares. Uno de estos métodos es el de Mansur y colaboradores, que se basa en mediciones espectrofotométricas de los filtros orgánicos extraídos con etanol. Las absorbancias de los extractos son medidas en el rango de 290 a 320 nm con intervalos de 5 nm. En este método el factor de protección solar (FPS) es calculado con una ecuación que relaciona los valores de absorbancia con los respectivos efectos eritémicos a la longitud de onda utilizada en cada medición. En este trabajo se analizaron tres bloqueadores con el método Mansur, obteniéndose valores de FPS mucho menores a los declarados. Este resultado motivó un análisis más minucioso que determinó que el método Mansur no es adecuado para bloqueadores con FPS mayor a 15. El análisis incluyó revisiones de los datos de FPS reportados por diversos autores usando el mismo método. Este trabajo también incluye la optimización de un ensayo simple que usa diluciones seriadas de cultivos de levadura para comparer, muy eficientemente, los efectos fotoprotectores de los bloqueadores solares. Este ensayo con levaduras permite comparar los efectos fotoprotectores en un amplio rango de FPS y puede incluir lociones comerciales, soluciones de extractos naturales, y activos en suspensión o solución.
Palabras clave: Factor de protección solar, FPS, radiación UV, ecuación Mansur.
INTRODUCTION
The demand for topical sunscreens increases each year as the recommendations to protect our skin from sun exposure become more widespread. These recommendations are based on the demonstrated correlation between extended exposure to the solar ultraviolet (UV) radiation and the occurrence of skin damage, premature aging, and skin cancer1. This problem is particularly critical in countries located close to the equator like Peru, and this was one of the main motivations for this work.
The UV radiation that reaches the earth surface is composed of 95% UVA (320 - 400 nm) and 4% UVB (280 - 320 nm) radiations. UVB is the predominant cause of erythema or sunburn and DNA damage, due to the formation of pyrimidine dimers. On the other hand, UVA is more related to tanning and photoaging but can also cause DNA damage indirectly through the formation of reactive oxygen species. UVC radiation (200 - 280 nm) is not normally present on the earth surface, except at regions of very high altitude2.
Because of their increasingly important functions, it is critical that the general public understands the degree of protection against UVA and UVB that sunscreens are able to provide. Currently, nearly sixty active compounds are allowed in the making of sunscreen products. These are divided into two main categories, depending on their physicochemical properties and mechanisms of action: The organic filters act by absorbing the UV radiation; and the other group mainly formed by inorganic compounds, act by reflecting or dispersing it1,3,4.
Regarding the parameters used to measure and define sunscreen efficiency, the term "sun protection factor" (SPF) is the most widely known and applied. The SPF of a sunscreen is measured in a laboratory. It is defined as the amount of UV radiation (exposure time) needed to produce a sunburn (erythema) on skin protected with a sunscreen, relative to that of unprotected skin1-4.
The standard method for SPF determination is based on the in vivo measurement of the minimal erythemal dose (MED) on volunteers with and without sunscreen application. However, this method is not devoid of flaws since some reports have demonstrated that it is unreliable to determine an SPF on the basis of a single assay. For example, a study performed on sixty different sunscreen products found that the discrepancies in the found and claimed SPFs were significantly greater when testing products of higher SPFs5. Furthermore, the standard in vivo assay is not only difficult and costly to implement but also and importantly, requires irradiation of small areas of the skin of volunteers. This fact raises some ethical implications that need to be considered.
There are several in vitro methods that have proved efficient and are widely used, but require specialized equipment and materials. Most of these methods are based on the spectrophotometric analysis in the 290 - 400 nm range of solid artificial substrates on which the sunscreen is spread. The substrate most favored is made of polymethylmethacrylate (PMMA)6. This method cannot be applied without these substrates and a specialized spectrophotometer. For this reason, it has been important to count on more simpler methods to quantify the photoprotective capabilities of sunscreens for research, regulatory, or consumer information purposes. One such method was reported by Mansur et. al. in 1986, which involves a simple UV-spectrophotometric assay of alcohol extracts of commercial sunscreens7, and has been used in several studies8-13.
The yeast Saccharomyces cerevisiae has long been used to study the responses to DNA damage caused by UV irradiation. Yeast cells can be easily cultured and therefore, offer multiple advantages as a testing system for the photoprotection capabilities of sunscreens. For example, yeast has been used to demonstrate the significant higher protective effects of the widely used sunscreen benzophenone, over its deleterious effects due to production of reactive oxygen species elicited by UV irradiation14.
The aim of this work was to compare the photoprotective effects of commercial sunscreens using two methods: The spectrophotometric assay proposed by Mansur et al., and one based on the survival rates of yeast cells upon exposure to UV radiation. The latter, while not a quantitative assay, could be used to simply and accurately compare the photoprotective effects of commercial sunscreens in their terminated form, filter solutions or lotions, and natural product extracts.
EXPERIMENTAL PART
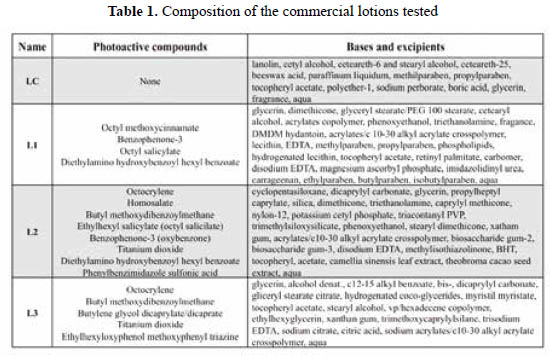
Sunscreens and control lotions and gels. Four lotions were purchased in different stores in the city of Lima. One is a moisturizing lotion (used as a control, "LC"), and the other three were sunscreens with claimed SPF values of 50 or 60 ("L1", "L2", "L3"). The declared lotion compositions are listed in Table 1.
Additionally, two gels were manufactured for some of the yeast assays (see below), one was a control ("GC") and the other had an SPF of 30 ("G30"). "GC" included the base Gransil EP-9® and excipients, while "G30" also included octocrylene, homosalate, benzophenone-3, and avobenzophenone.
Spectrophotometric assays. The method described by Joao De Souza Mansur et. al. was used to determine the SPF values7, with a few modifications as described. Whenever possible, all suspensions and solutions were kept protected from light until their immediate use. For each lotion sample, 1,0 g was weighed, transferred to a 100 mL volumetric flask, and mixed with 80 mL ethanol on a rotatory shaker for 45 min. Enough ethanol was added to complete the volume and the suspension was well mixed. An aliquot of each suspension was centrifuged at 13700xg for 5 minutes. 150 µL of the clear supernatant were diluted to 25 mL with ethanol, thus obtaining a lotion solution with a final concentration of 0,06 mg/mL. This concentration is lower than that called in the original protocol of 0,2 mg/mL but was preferred to allow absorbance readings below 0,800. Thus, to apply the denoted "Mansur equation" for the estimation of the SPFs, a dilution factor of 3,333 was applied on all the absorbance values. A Biomate 3 spectrophotometer (ThermoFisher) was used to measure absorbances in the 290 – 320 nm range (with 5 nm intervals), using ethanol as blank. All the extractions and absorbance measurements were repeated independently at least three times, and the averages were used to apply Mansur equation7:
Where: CF is the correction factor (=10); "EE", the erythemal effect of radiation at wavelength λ; "I", the solar intensity spectrum; and "ABS", the absorbance. "EE", "I", and "ABS" are values obtained or applied for every wavelength (λ). The values for each of the [EE(λ)xI(λ)] products have been reported by the authors as normalized on the basis of the work by Sayre et. al., and are: 0,0150 for 290nm; 0,0817 for 295nm; 0,2874 for 300nm; 0,3278 for 305nm; 0,1864 for 310nm; 0,0839 for 315nm; and 0,0180 for 320 nm7,15.
Spectrophotometric assays using homosalate. Homosalate (Salisol®) was obtained from "Salicylates and Chemicals" (Mumbai, India). To apply the protocol described by Mansur et al. and in order to obtain absorbance values within the acceptable range of 0,200 to 0,800, the following modifications were adopted: An 8 % w/w homosalate solution was diluted weighing 1,5 g and adding ethanol up to 50 mL. This solution was diluted again, measuring 200 µL and adding ethanol up to 25 mL. With this final dilution, the prepared solution contains the original 8 % w/w standard solution with a concentration of 0,24 mg/mL instead of 0,2 mg/mL, which is the concentration obtained when following Mansur’s protocol for the sunscreen lotions. Therefore, the dilution factor 0,8333 was applied to all absorbance values obtained before applying Mansur equation.
Yeast strains and culture conditions. The yeast Saccharomyces cerevisiae strain used for all assays was of the W303 background (MATa leu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15)16. Yeast cells were grown on YPD (1 % yeast extract, 2 % peptone, 2 % glucose) broth, or solid media containing 2 % agar. All cultures were grown at room temperature, with rotating agitation for liquid cultures, for 2 to 3 days.
In vivo photoprotection assays using Saccharomyces cerevisiae. Yeast cells were grown on rich YPD broth with shaking and at room temperature for three days. Aliquots of these cultures were spread onto YPD plates using sterile glass beads to obtain a uniform distribution of cells. For the serial dilution assays, the three day cultures were briefly sonicated to disrupt cell aggregates and serially diluted with sterile water to obtain 100, 500 and 2500 fold dilutions. 4 µL of each dilution were spotted in two rows on sections of YPD plates, and the spots were allowed to dry. Each plate was covered with a cellophane sheet similarly divided in sections, each of which was covered with suspensions of the lotions or gels to be tested. These suspensions were made weighing 2 g of the gel or lotion, and adding enough water or alcohol as suitable to produce a suspension that is easily spreadable on the cellophane sheet. The area of each cellophane section was estimated so that to have spread on it a volume of the suspension to produce a layer of 2 mg/cm2 of the lotion or gel, to mimic the conditions recommended for sunscreen application on the skin prior to sunlight exposition1.
For UVB irradiation, the source was a VWR transilluminator (VWR International, U.S.A.) with four 8W light tubes that emit 302 nm radiation. The transilluminator was positioned 10 cm above the plate covered with the cellophane sheet so that the UVB radiation impacted directly on it for 2 minutes, at maximum intensity.
For UVC irradiation (254 nm), the cellophane-covered plates were positioned at the base of a wood chamber that included two GE G15T8 germicide lamps (UV radiance RG-3, 4,9W) located 43 cm above. These exposures were for one minute.
RESULTS AND DISCUSSION
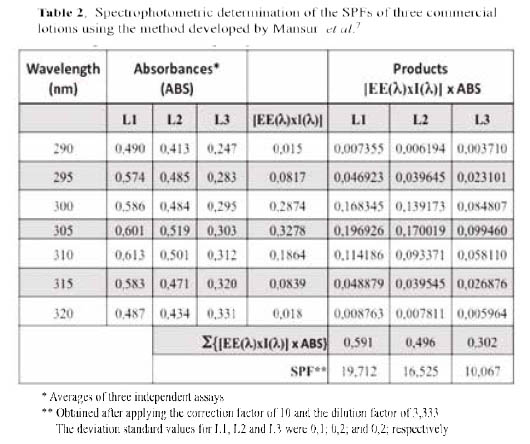
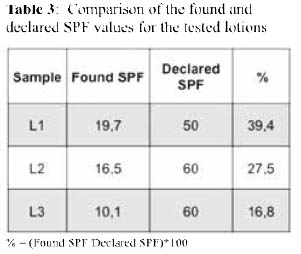
Spectrophotometric assays. The method described by Mansur et al., was applied to determine the SPF values of three commercial lotions (denoted L1, L2, and L3) and the results are shown in Table 2. Surprisingly, all found SPF values were markedly lower than those expected (declared), ranging from 16,8 to 39,4 % (Table 3).
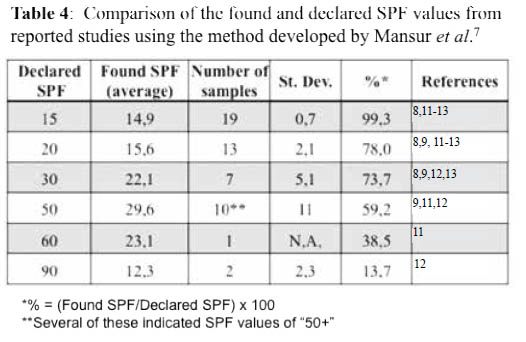
These findings prompted a more thorough analysis on reported studies that had used the Mansur method to quantify the SPFs of commercial sunscreens 8,9, 11-13, and the results are listed in Table 4. All reported results were compared with the respective declared SPF value for each sunscreen lotion tested. While there was a good agreement for the lotions with SPF 15, the found values started differing considerably as the declared SPF values increase. For lotions of SPFs higher than 50, the found values corresponded to only the 13,7 - 59,2 % of those declared.
Altogether, these results suggest that the Mansur method is not suitable to assay sunscreens of SPF values above 15. However, this easy and simple method has been used by several investigators to determine the SPF values of extracts from natural products or lotions prepared with these, and commercial sunscreens8-13.
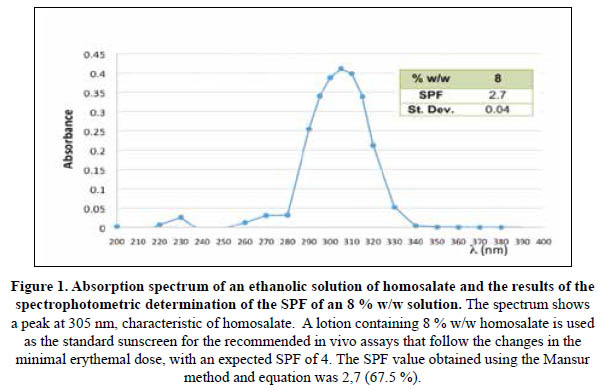
The list of standard sunscreen lotions recommended by the Food and Drug Administration (FDA) and other international organizations includes 8% w/w homosalate, to provide an SPF value of 4,017. For this reason, Mansur et al. used this standard lotion to develop their equation. Therefore, given the discrepancies in the results and to confirm the adequacy of the followed procedure, the spectrophotometric assays were also run using an 8 % w/w homosalate standard solution, and the results are shown in Figure 1. While the absorption spectrum shows an optimum wavelength of 305 nm, similar to that reported by the manufacturer of 307 nm, the SPF value obtained was also significantly lower than the expected value of 4,0. Nevertheless, the UV specific extinction value obtained was close to that reported in the certificate of analysis received, proving that the absorbance readings and calculations applied were correct.
One explanation for the discrepancy could be the fact that Mansur et al. used a homosalate standard lotion for the development of their equation. It is then possible that one or more of the excipients had been extracted into the ethanolic solution and contributed to the obtained absorbances in the 290 – 320 nm range, thus causing the overestimation of the SPF values. The concentrations of those excipients remain basically unchanged during the manufacturing of higher SPF sunscreens and therefore, do not proportionately produce that overestimation. Accordingly, when applying the Mansur method and equation to determine the SPFs of high SPF sunscreens, the obtained values are lower.
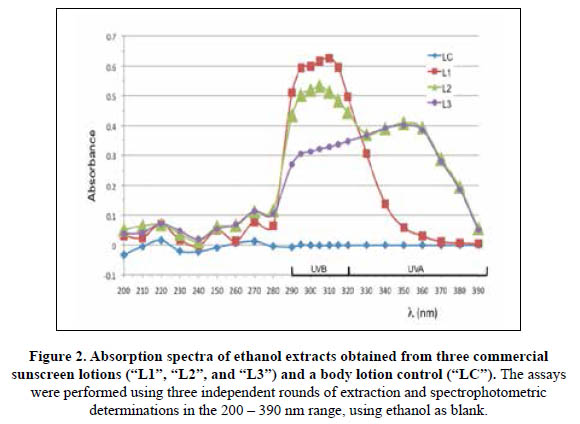
On the other hand, the labels for the tested lotions L1, L2 and L3 indicated they all included UVA and UVB sunscreen actives, as most modern sunscreens do1. In order to determine if these photoactive compounds were efficiently recovered with the ethanolic extractions, the lotions were processed as described, and the absorbances of the final solutions were measured in the 200 – 390 nm range. The absorption spectra shown in Figure 2 indicated that both UVA and UVB filters were recovered for L2 and L3 but not for L1. The ethanolic extract of the latter exhibited the highest absorbances in the UVB range but the lowest values for wavelengths above 330 nm (UVA range). Accordingly, the found SPF for L1 was closer to its declared value than for the other two lotions (Table 3), since only the absorbances for the UVB range are taking into account for its estimation using the Mansur method. Therefore, this simple spectrophotometric method is not only inaccurate and inefficient but also, does not comprise the photoprotective effects of UVA actives such as butyl methoxydibenzoylmethane (avobenzone, Parsol 1789), which is included in both L2 and L31,3.4. Importantly, filters such as titanium dioxide and zinc oxide are not soluble in ethanol and thus, their photoprotective effects are also not taking into account when using the Mansur method.
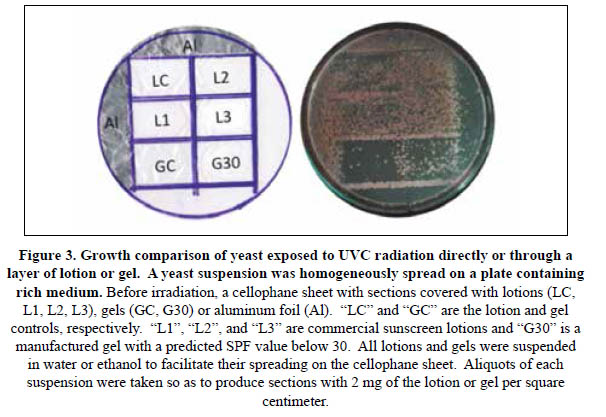
In vivo photoprotection assays using yeast cells. The results shown above prompted the search for a more reliable, simple and efficient means to demonstrate and compare the photoprotective capabilities of sunscreen lotions. Thus, a commonly used method based on the survival rates of cultures of the yeast Saccharomyces cerevisiae was optimized and adapted for this purpose. First, plates containing solid rich medium covered with homogeneous layers of yeast cells were used. Before irradiation, each plate was covered with a cellophane sheet divided in small sections on which, aliquots of lotion or gel suspensions had been homogenously spread. Besides these and to serve as controls, parts of the cellophane sheet were covered with aluminum foil (to provide a shield against the UV radiation), and others were left uncovered (unprotected control). The amount of suspension spread was estimated so that to achieve layers of 2 mg of the gel or lotion per square cm (2 mg/cm2), the thickness of sunscreens that is recommended for adequate protection of the skin1 (Figure 3). The cellophane-covered plates were irradiated with UVC radiation for 1 minute, as described.
This procedure allowed only a qualitative comparison of the photoprotective effects, but it was possible to clearly distinguish between the sections that were left unprotected (no growth) and those that were less or more protected (lotion and gel sunscreens and controls, or aluminum foil). The SPF-30 gel (G30) allowed formation of a higher number of colonies than its respective control (GC). It was also possible to clearly distinguish higher photoprotective effects for the L1, L2 and L3 sunscreens than for the lotion control (LC).
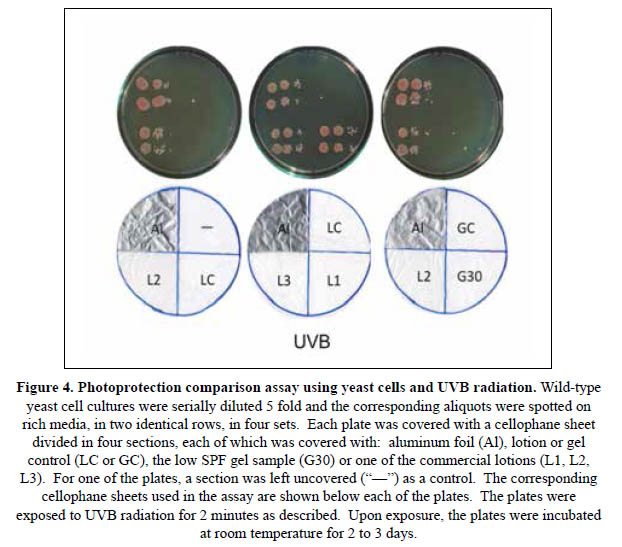
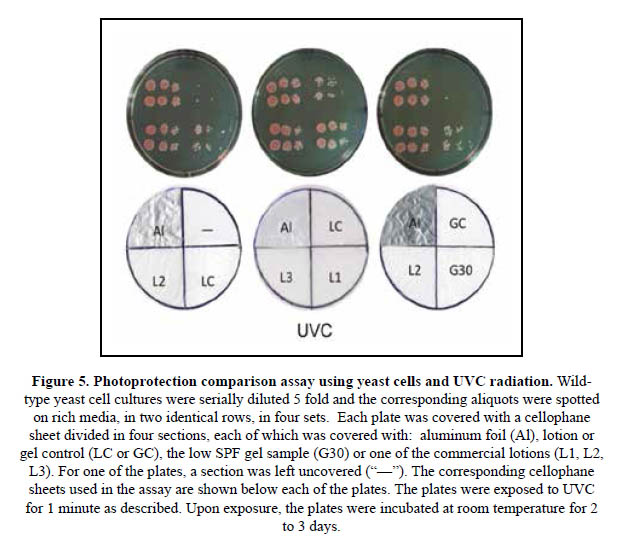
In order to improve the resolution of the assay, the method was modified using small aliquots of 5 fold serial dilutions of yeast cultures instead of the yeast layers (Figures 4 and 5). In these assays, the rich media on the plates were spotted with the same three serial dilutions in two identical rows, on each of four sections. On the other hand, a cellophane sheet divided in four sections was also prepared to cover each plate. In each of the cellophane sheets, one of the sections was covered with a piece of aluminum foil, a lotion or gel suspension to produce a thickness of 2 mg/cm2, or left unprotected. The cellophane-sheet covered plates were then irradiated as indicated.
With this simple assay it was possible to efficiently compare the photoprotective effects of the lotions or gels on the yeast cells spotted on the plate, within the range limited by the totally exposed and unprotected sections ("–"), and the fully protected ones covered with aluminum foil ("Al").
Figure 4 shows a representative set of results for an assay of photoprotection against UVB radiation. Here, the lotion control provided no protection, with the spotted cells showing lack of growth, similar to the unprotected section. Meanwhile, the photoprotective effects of the L1, L2 and L3 sunscreens are evident since yeast cells are able to show robust growth in the respective sections, while no colony was formed in the LC or unprotected section. Comparing the UVB photoprotective effects of the lotions, it is possible to conclude that L1>L3>L2>>>>>LC. Under these assay conditions, yeast cells were not able to survive when UVB irradiated under the sections covered with the G30 or GC samples. It is important to note here that the SPF values obtained using the Mansur method (Table 2) are 19,7, 16,5 and 10,1 for the L1, L2 and L3 lotion, respectively. Similarly, the absorption spectra shown in Figure 2 suggest that the UVB photoprotection effects for L3 are considerably lower than L2. The yeast assay indicates the opposite, providing more evidence for the inadequacy of the spectrophotometric method.
Figure 5 shows results obtained following the same procedure except that the cellophane- covered plates were exposed to UVC. Here, lotions L1, L2 and L3 showed similar levels of strong protection. On the other hand, a small number of colonies were able to form in the areas covered with LC and G30 but no growth was evident in the GC and unprotected sections. This high protection level correlates better with the declared SPF values for the lotions. It is important to note that the protective effects of titanium dioxide and zinc oxide filters are more evident against UVC, and this fact is probably the reason behind the robust growth observed in the L1, L2 and L3 sections. The G30 gel lacks both of these filters.
Besides providing a very simple and efficient means to compare the photoprotective effects of sunscreens, the described yeast assay offers the opportunity to show in a very didactic manner, the benefits of sunscreen usage. More simpler versions of this assay have already been implemented in school exercises in the U.S.A. that allow the students to learn firsthand about the dangers of sun exposure without adequate photoprotection18. Therefore, this yeast assay could be efficiently used not only to compare sunscreens in an academic or regulatory setting, but also to educate the general public about the imperative need to protect oneself from the dangerous effects of UV radiation.
CONCLUSION
This study demonstrates that the denoted "Mansur method" and "Mansur equation" should not be used to assay sunscreens of SPFs above 15. Further, a yeast assay is described that could be used to simply and reliably compare the photoprotection levels conferred by sunscreens.
ACKNOWLEDGEMENTS
We thank "InnovatePerú" (Contract N°157-PNICP-PIAP-2015) and the Research Support Office at the "Universidad Nacional Agraria La Molina" (Grant for scientific and technological research - UNALM 2013) for providing the funding that made this work possible. We also thank Mr. José Kitazono and Ms. Bélgica Pérez for their help throughout these studies.
REFERENCES
1. Jou PC, Feldman RJ, Tomecki KJ. UV protection and sunscreens: What to tell patients. Cleve Clin J Med. 2012;79:427-436. [ Links ]
2. Diffey BL. What is light? Photodermatol Photoimmunol Photomed. 2002;18:68-74. [ Links ]
3. Jansen R, Wang SQ, Burnett M, Osterwalder U, Lim HW. Photoprotection: part I. Photoprotection by naturally occurring, physical, and systemic agents. J Am Acad Dermatol. 2013;69:853 e1-12. [ Links ]
4. Jansen R, Osterwalder U, Wang SQ, Burnett M, Lim HW. Photoprotection: part II. Sunscreen: development, efficacy, and controversies. J Am Acad Dermatol. 2013;69:867 e1-14. [ Links ]
5. Miksa S, Lutz D, Guy C. Relevance of Sun Protection Factor claim: Review of a study with 60 different commercial sunscreen products from European market. Household Pers. Care Today. 2016;11:64-68. [ Links ]
6. Cole C. Sunscreens--what is the ideal testing model? Photodermatol Photoimmunol Photomed. 2014;30:81-87. [ Links ]
7. Mansur JS, Breder MNR, Mansur MCA, Azulay RD. Determinação do fator de proteção solar por espectrofotometria. An Bras Dermatol. 1986;61:121-124. [ Links ]
8. Dutra EA, Oliveira DAGdCe, Kedor-Hackmann ERM, Santoro MIRM. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev Bras Cienc Farm. 2004;40:381-385. [ Links ]
9. Fonseca AP, Rafaela N. Determination of Sun Protection Factor by UV-Vis Spectrophotometry. Health Care: Curr Rev. 2013;1:108 e1-e4 [ Links ]
10. Inocente-Camones MÁ, Tomas-Chota GE, Huamán-Malla J, Muñoz-Jáuregui AM, García-Morán RI, Quispe-Fuentes G, et al. Actividad antioxidante y fotoprotectora in vitro de una loción y gel elaborados con extracto estabilizado de camu camu (Myrciaria dubia, Kunth). Rev Soc Quim Peru. 2014;80:65-77. [ Links ]
11. Mbanga L, Mulenga M, Mpiana PT, Bokolo K, Mumbwa M, Mvingu K. Determination of Sun Protection Factor (SPF) of Some Body Creams and Lotions Marketed in Kinshasa by Ultraviolet Spectrophotometry. Int J Adv Res Chem Sci. 2014;1:7-13. [ Links ]
12. Omar KA, Abdulrahman RS. Determinations of Sun Protection Factor (SPF) of some sunscreens marketed in Kurdistan Region by UV-Visible spectrometry and study their Rheological properties. Int J Pharm Chem. 2015; 05:40-44. [ Links ]
13. Sudhahar V, Balasubramanian V. Sun production factor (SPF) determination of marketed sunscreen formulation by in-vitro method using UV-VIS spectrophotometer. Arch Appl Sci Res. 2013;5:119-122. [ Links ]
14. Beckett A, Mcclure B, Zimmerman K. Benzophenone and Padimate-O Protect Saccharomyces cerevisiae From UV Radiation and Cause Little Harm From UV- Induced Reactive Chemical Species. J Exp Microbiol Immunol. 2004;5:37-43. [ Links ]
15. Sayre RM, Agin PP, Levee GJ & Marlowe E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem and Photobiol. 1979; 29: 559-566. [ Links ]
16. Thomas BJ, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619-630. [ Links ]
17. Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use - Testing procedures. United States: FDA; 2016. [ Links ]
18. Association for Biology Laboratory Education (ABLE). Tested Studies for Laboratory Teaching. En: D´Costa AR, Santoro I, editors. 31st Annual ABLE Conference. Proceedings of the 30th Workshop/Conference of the Association for Biology Laboratory Education (ABLE); June 9-13, 2009; University of Delaware Newark, DE: K.L. Clase; 2009. P. 371-382 [ Links ]
Recibido: 21-04-17
Aprobado: 13-09-17