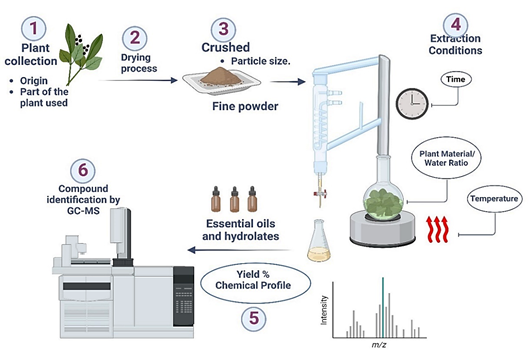
1. Introduction
Essential oils (EO) are natural volatile liquid mixtures of hydrophobic nature. They are usually complex and consist of low molecular weight compounds (Busatta et al., 2017). These molecules are synthesized as secondary metabolites and handle the distinctive odor of plants, often possessing medicinal properties and high commercial value (Lo et al., 2020; Bhavaniramya et al., 2019). Essential oils are obtained from various plant materials, including flowers, shoots, leaves, seeds, fruits, roots, twigs, barks, herbs, and wood, and they constitute a complex mixture of hydrocarbons, alcohols, esters, aldehydes, carboxylic compounds, and, in some cases, phenylpropanoids (Abbas et al., 2022). The most frequent hydrocarbons are terpenoid compounds, but sesquiterpenes can also be found (Caputo et al., 2022; Busatta et al., 2017). EOs protect plants from viral, fungal and bacterial diseases; they also prevent oxidative damage to many cell structures caused by ultraviolet radiation (Önder et al., 2024). EOs are among the most important products in both the agricultural and food industries (Oliveira et al., 2021; Falleh et al., 2020). There are an estimated 3,000 known essential oils, of which about 300 are commercially relevant. (Sharmeen et al., 2021). They are commonly used as flavoring agents in food and beverage products (De Cicco et al., 2023; Saeed et al., 2022), as well as in the manufacture of perfumes, pharmaceuticals, and cosmetics (Sharmeen et al., 2021; Brito et al., 2021). Furthermore, natural compounds can play an essential role in mitigating antimicrobial resistance in foodborne pathogens (Peralta-Canchis et al., 2024). The potential of EOs as antibacterials and herbicides has also been identified, as they are biodegradable, have a high structural diversity and can reduce natural weed resistance (Yeddes et al., 2022). The synergies between the different compounds in EOs enable them to exhibit these properties (Karalija et al., 2020; Rafya et al., 2024).
EOs are a complex mixture of compounds, mainly monoterpenes, sesquiterpenes, and their oxygenated derivatives (alcohols, aldehydes, esters, ethers, ketones, phenols, and oxides) (Popa et al., 2021). Some volatile compounds include phenylpropenes and specific sulfur - or nitrogen - having substances (Badawy et al., 2014). Generally, the essential oil composition is a balance of several compounds, although in many species, one constituent may prevail over all others (Popa et al., 2021; Badawy et al., 2014).
Essential oils are commonly obtained by steam distillation or hydrodistillation, and at the end of these processes, hydrosols or hydrolats are obtained as an aqueous fraction that is separated from the EOs (Brito et al., 2021; Stratakos et al., 2016). Several different extraction techniques are widely used for EO, such as steam distillation and solvent extraction (Noori & KhajeNoori, 2013). These methods usually have some drawbacks such as low extraction efficiency and selectivity, the use of large amounts of solvents, and long extraction times (Yeddes et al., 2022; Rafya et al., 2024). In many cases, the quality of the essential oil obtained by conventional methods can be influenced by hydrolysis or oxidation that may take place due to the long extraction time and/or high amount of water (Stratakos et al., 2016; Önder et al., 2024).
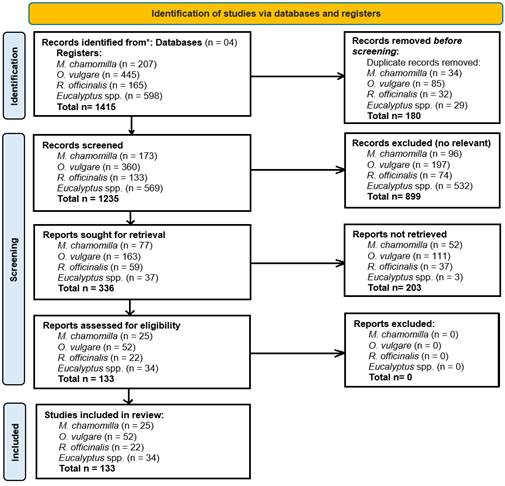
Figure 2 PRISMA flow chart for studies related with extraction of essential Oils by Hydrodistillation of M. chamomilla, O. vulgare, R. officinalis and Eucalyptus sp. (Page et al., 2020).
The present review aims to update the information related to the extraction of essential oils by Clevenger-type hydrodistillation of four aromatic plant species of high commercial value: Matricaria chamomilla (MC), Rosmarinus officinalis (RO), Origanum vulgare (OV), and Eucalyptus spp. (EU), with the objectives of evaluating the results of original articles regarding the origin and characteristics of the plant material, the conditions for extraction, as well as the yield and metabolites obtained by these studies.
2. Methodology
The review follows the PRISMA guidelines for scoping reviews (PRISMA-ScR). Access to the information and data obtained in this research is available full text in Open Access on the Zenodo portal (Figure 2).
A bibliographic search was performed in the databases Scopus, Web of Science, PubMed, and Google Scholar for a period of 10 years (2013-2023). The names of the plant species, "Matricaria chamomilla", "Origanum vulgare", "Rosmarinus officinalis" and "Eucalyptus" were used as keywords. Additionally, words such as "Essential oils" and "Hydrodistillation," along with the Boolean operators "OR", "AND" or "NOT," were used as connectors for the search (Supplementary Material).
3. Extraction of essential oils
3.1 Hydrodistillation process
Hydrodistillation (HD) is the simplest and oldest method for obtaining essential oils, and the Clevenger-type HD system is recommended in the third edition of the European Pharmacopoeia for the determination of EO content (El-Assri et al., 2021; Falleh et al., 2020). HD is a common method that involves the evaporation of volatile plant components at a lower temperature in the presence of steam; for this, a mixture of water and dried plant material is brought to a boil in a previously established proportion (Berechet et al., 2017). The essential oil-laden vapors pass through the coolant and then condense, later recovering in a burette to be separated from water; the distillate holds both the hydrolate and EOs (Lo et al., 2020). HD is considered the most widely used method for the extraction of essential oils from plants. Although widely used, this extraction technique is more time-consuming and can lead to the degradation of the more thermolabile molecules (Hashemi et al., 2017).
Although HD has disadvantages such as long extraction times and low yield, requiring a larger amount of fresh plant material (Chávez et al., 2016), when comparing HD with advanced extraction techniques (microwave-assisted, supercritical, etc.) (El-Assri et al., 2021). HD is still the most common industrial extraction approach for EOs. This is due to its simple installation (requiring no expensive equipment), ease of implementation, and selectivity (Gladikostić et al., 2023; Bhavaniramya et al., 2019). Therefore, HD remains important for industrial applications (Busatta et al., 2017).
3.1. Plant material
3.1.1. Origin of plant material
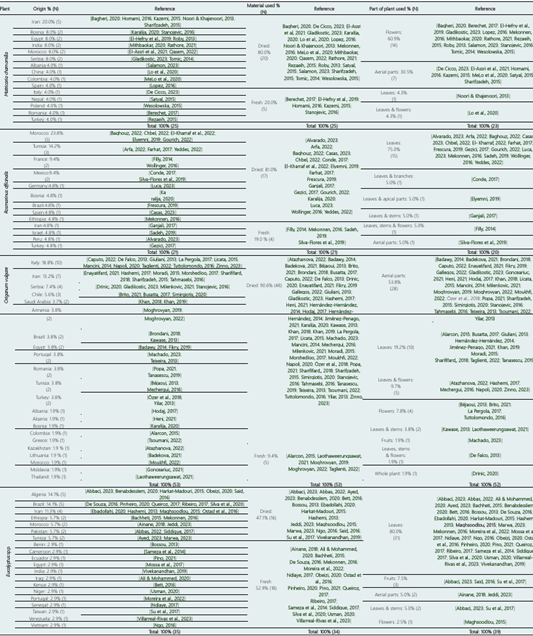
The origin of the plant species M. chamomilla, R. officinalis, O. vulgare, and Eucalyptus spp. is described in Table 1, while the geographical distribution is shown to Supplementary Material.
M. chamomilla (Chamomile) is a native plant to southeastern Europe and northwestern Asia but can also be found in many countries and has been introduced naturally in Great Britain, Australia, and North America (El Mihyaoui et al., 2022). Most of the studies reviewed on this species in this research are originates mostly from the Middle Eastern region, Balkan countries, Mediterranean Europe, Asian countries and South America. The origin of MC is important because exposure of chamomile flowers to cold stress (0 to +10 °C) has been reported to negatively affect the yield of oil extraction from the flowers (Bagheri et al., 2020).
Rosmarinus officinalis L. (Rosemary) is a perennial plant and a member of the Lamiaceae family, distributed throughout the Mediterranean countries, where it is primarily used in food and as a raw material for obtaining essential oils (Sadeh et al., 2019). Table 1 shows that the countries with the highest contribution of plant material for the primary studies of this review were from North Africa, Other countries contributing plant material were from the Middle Eastern and European countries; among the countries in the Americas were Mexico (9.4%), Brazil, and Peru (4.8%).
Origanum vulgare L. (Oregano) is an important species due to its antibacterial, antifungal, antioxidant, antiviral, and flavoring properties supported by its complex chemical composition (Béjaoui et al., 2013). OV is a perennial herbaceous species of Mediterranean origin from the Lamiaceae family, known and used since ancient times (Gonceariuc et al., 2021).
As shown in Table 1, OV has a more diverse geographic distribution, with most of the plant material used in the research originating from countries such as Italy (18.8%), other European Mediterranean countries, and the Balkan Peninsula. However, the second largest country of origin is Iran (13.2%), along with other Middle Eastern countries.
Table 1 Origin and characteristics of plants used in the studies on essential oil extraction by hydrodistillation for species M. chamomilla, O. vulgare, R. officinalis, and Eucalyptus spp.
Note: %(N) = Percentage% (Number of studies).
Asian countries as well as American countries have contributed plant material for studies of this species in smaller quantities.
The genus Eucalyptus is the subject of numerous studies due to its wide geographical distribution and adaptability to different climatic conditions (Ebadollahi et al., 2020). Plant materials of this species have been found on several continents and in various countries. In the Americas, research has predominantly focused on Brazil, Venezuela, and Ecuador. In Africa, studies were conducted in Algeria, Tunisia, Ethiopia, Morocco, Benin, Egypt, Kenya, Niger, Senegal, and Cameroon. In Asia, these include Iran, Pakistan, Taiwan, India, Iraq, and Vietnam. Finally, in Europe, only one study was found in Portugal.
3.1.2. Parts of the plant used for essential oil extraction
Table 1 shows the percentages according to the parts of the plant used for the extraction of the essential oil of the 4 plant species (MC, RO, OV, and EU). For MC, it is seen that the most used part was only flowers (60.9%), followed by a mixture of aerial parts (30.5%), only leaves (4.3%), and a mixture of leaves and flowers (4.3%). Earlier research reports that in addition to flowers, leaves, stems, and roots of the plant also contain essential oil (Singh et al., 2011). Compounds such as (Z)-3-hexenol, (E)-β-Farnesene, α-farnesene, germacrene D, (E)-nerolidol, Linalool, Geraniol, β-Elemene, α-Spatulenol, τ-Cadinol, τ-Muurolol, β-Caryophyllene, cis-Caryophyllene, Caryophyllene oxide, and Chamomillol have been found. However, these oils lack Chamazulene, and α-Bisabolol oxides were present as minor components (El Mihyaoui et al., 2022).
Moreover, RO is a plant from which essential oils are primarily extracted from leaves (75.0%), with a much smaller proportion coming from a mixture of leaves and stems, apical parts, branches, flowers, or aerial parts (5.0% in all cases). RO leaves are known to be rich in bridged bicyclic monoterpenes such as α-pinene and β-pinene, as well as their enantiomers limonene and linalool, so it would be logical to use the leaves of the plant for the extraction of essential oils in most studies (Mekonnen, 2016).
In the case of OV, the parts used were more varied, with the highest usage being a mixture of aerial parts (53.8%), followed by leaves only (19.2%), a mixture of leaves and flowers (9.7%), only flowers (7.8%), a mixture of leaves and stems (3.8%), a mixture of fruits and cuttings (1.9%), a mixture of leaves, stems, and flowers (1.9%), and the whole plant (1.9%). It has been reported that monoterpenoid chemical compounds of the essential oils of this plant species, such as thymol, carvacrol, limonene, myrcene, among others, are widely distributed throughout the plant, so it would be right to use the aerial parts of the plant (Atazhanova, 2022). In EU, the most considered plant sample is also the leaves (80.0%); fruits (7.7%); aerial parts; leaves and stems (5.0% in both cases). This would be explained by the reported composition of oxygenated monoterpenes and sesquiterpenes compounds, with the main components being 1,8-Cineole (carvacrol), Spathulenol, α-Terpineol, among others (Harkat-Madouri, 2015).
3.1.3. Conditioning of plant material
To ensure mass transfer enhancement, plant material should be crushed to a suitable particle size, typically ranging from 100 µm to 2 mm (Gladikostic et al., 2023). Larger contact surfaces are known to increase extraction efficiency (Abbas et al., 2022). Furthermore, finer particles enhance the rate of mass transfer from the solid to the liquid phase (Moreira et al., 2022). Therefore, it is recommended that the diameter of the plant material be no larger than 2 mm and no smaller than 0.5 mm (<10%) (Gladikostic et al., 2023). In this review, all the articles reported that the material was crushed prior to the HD process.
Drying is a traditional preservation method for food in general and medicinal plants in particular (Abbas et al., 2022). This method is based on simultaneous heat and mass transfer phenomena and is widely used to preserve properties such as aroma, flavour and nutritional factors (Caputo et al., 2022). The aim of drying is to decrease the weight of the plant raw material without affecting its quality. The drying treatment is essential for the processing of aromatic and medicinal plants (Brondani et al., 2018), as it slows down the growth of microorganisms and inhibits biochemical reactions that can affect the organoleptic properties, thus increasing the shelf life of the products (Abbas et al., 2022; Ozdemir et al., 2018). A study into the effects of hot air drying on OV and RO, the results showed that hot air caused drastic losses in the oregano plant as the essential oils volatilized and the dried product lost quality, while the volatile compounds in the rosemary plant were not affected by hot air drying (Ozdemir et al., 2018). This study found that different drying methods led to variations in the proportions of essential oil constituents, with new compounds appearing and others disappearing. Further research showed that oxygenated sesquiterpenes increased to varying degrees after drying, except for a slight decrease after oven drying (Brondani et al., 2018).
Table 1 shows that dried plant material was the most used in the extraction of AEs from MC, RO, and OV (80.0%, 81.0%, and 90.6%, respectively), unlike EU, where dried material was used in only 47.1% of the articles reviewed in this research. Is important to remember that drying methods should be selected considering the active metabolites, plant species, and plant tissues where these molecules accumulate (Caputo et al., 2022; Ozdemir et al., 2018). The drying process has been reported to have a great influence on the chemical constituents and yield of the essential oil of aromatic plants (Bathily et al., 2023). In a study on the effects of hot air drying on OV and RO, the results showed that hot air caused drastic losses in the OV as the essential oils volatilized and the dried product lost quality. In contrast, the volatile compounds in the RO were not affected by hot air drying; the difference in drying method led to variations in the proportions of essential oil components, as well as the appearance of new compounds and disappearance of others (Ozdemir et al., 2018). In another study, it was shown that oxygenated sesquiterpenes increased to varying degrees after drying, except for a slight decrease after oven drying (Brondani et al., 2018).
In general, oven drying is considered the best method according to previous studies; however, it is important to establish the drying conditions for each aromatic species, as applying the optimal drying method can be useful in increasing the composition and yield of the essential oil (Özer et al., 2018). In addition, air drying under sun or shade conditions is usually more economical; however, it requires prolonged times that lead to a loss of metabolites (Piri et al., 2019). Furthermore, being in ambient conditions, factors such as temperature and humidity are not controllable, increasing the probability of contamination of the plant material (Gourich et al., 2022). That is why other modern methods are often used to dry plants, such as oven drying, dehydration, and lyophilization (Abbas et al., 2022).
According to the type of drying, as shown in Table 1, the plant materials used were Fresh (20.0%) and Dried (80.0%) for MC; Fresh (19.0%) and Dried (81.0%) for RO. In the case of OV, it is seen that fresh material was considerably less used than Dried material (9.4% and 90.6%, respectively), unlike EU where fresh material represented 52.9% compared to dried material at 47.1% in the studies considered. The drying method, drying speed, and drying temperature have a significant impact on the quantity and quality of the active ingredients of aromatic plants (Piri et al., 2019). Despite technical advances, the choice of appropriate drying methods remains a fundamental economic and ecological criterion for the preservation of aromatic plants (Abbas et al., 2022). The drying methods recommended in the literature and the methods used in practice are different, confirming the need for research on this topic (Özer et al., 2018).
3.2. Hydrodistillation conditions of essential oils
The analysis of essential oils has shown that their chemical profile can differ not only in the number of different constituents but also in the structure of the extracted molecules, depending on the extraction method, which influences the characteristics of the essential oil (Figure 1).
It has been proved that various extraction methods can produce essential oils with a more natural organoleptic profile. This difference can be attributed to the varying composition of the oils depending on the conditions used for their extraction (Stratakos & Koidis, 2016).
3.2.1. Plant material/water ratio
Although the extraction of essential oil by HD appears to be a simple process, it has many drawbacks. During HD, plant materials are exposed to boiling water or steam to release the EO they contain through evaporation. As the steam and essential oil vapors condense, they are collected and separated.
Since the EO are exposed to boiling water for prolonged periods, by-products may form due to the amount or acidity of the water. This may lead to differences in the composition of the extracted EO (Stratakos & Koidis, 2016). Table 2 shows the plant material/water ratios reported by the studies that were part of this research.
The most used ratio for the extraction of the 4 plant species (MC, RO, OV, and EU) was one gram of plant material per 10 ml of water (1/10), with percentages of 36.3%, 37.5%, 36.1%, and 29.4% respectively for each plant species. The second most reported ratio was 1/15 for MC (18.2%); between 1/7 and 1/16 (12.5%) for RO; between 1/1 and 1/33 (7.1%) for OV; and 1/3.3 (17.7%) for EU. The determination of plant material/water ratio is important because during HD, hydrolysis of esters into alcohols and acids can occur, which can have significant consequences in the case of oils with excessive amounts of esters. Furthermore, some essential oils require rectification; this process involves redistillation of the oil to remove undesirable impurities (such as waxes among others), as well as components that may give it an unacceptable odor (Stratakos & Koidis, 2016).
Hydrolate (HY) is the fraction recovered from the aqueous distillate generated during HD. It is also known as recovered essential oil or water-soluble essential oil, composed of volatile compounds due to its isolation by a distillation-extraction process (Lei et al., 2018). The type and chemical composition of the HY from obtaining essential oils are precisely related to the amount of water used during extraction. GC-MS analysis and/or NMR spectroscopy have shown that non-polar molecules are the main components of essential oils, while more hydrophilic molecules and trace essential oils form HYs (Brito et al., 2021; Lei et al., 2018).
3.2.2. Hydrodistillation temperature
Temperature is one of the crucial parameters influencing the HD process. Increasing the extraction temperature beyond a certain threshold may lead to the degradation of essential oil components. It is advisable to determine the maximum extraction temperature for each plant material through experimental means (Noori & KhajeNoori, 2013). For essential oil extraction, the optimal conditions for HD usually occur at temperatures between 100 and 175 °C (Noori & KhajeNoori, 2013). Generally, the percentage of major components increases with rising temperature up to 150 °C; however, around 175 °C, the number of components decreases, and a burnt-smelling extract is produced, resulting from the degradation of some essential oil components (Noori & Khajenoori, 2013). In this study, the temperatures for the HD process are shown in Table 2. For MC, the HD temperature range was from 100 °C to 200 °C, with 100 °C being the most used temperature (50.0%), followed by 150 °C (33.3%), and 200 °C (16.7%). In RO, the temperatures used were 100 °C (66.7%) and 80 °C (33.3%). For OV, distillation temperatures ranged from 90 °C to 260 °C, with 220 °C being the most used temperature (22.3%), followed by 90 °C, 100 °C, 110 °C, 180 °C, 210 °C, 240 °C, and 260 °C (11.1% for all temperatures mentioned). For EU, only three research were found that reported the distillation temperature, with 100 °C representing 66.7% and one study using 120 °C (33.3%).
3.2.3. Hydrodistillation (HD) time
The study of HD times is extremely important since it is known that in many essential oil (EO) extraction processes, the quality of the EO obtained by HD can be influenced by hydrolysis or oxidation that can occur due to a long extraction time and/or a high amount of water used (Stratakos & Koidis, 2016). HD times for MC, OV, RO, and EU are shown in Table 2. For MC, HD times ranged from 2 to 12 hours, with the most used times being 3 hours (38.1%) and 4 hours (28.6%); followed by 2 hours (14.2%), 6 hours (9.5%), 10 hours, and 12 hours (4.8% each). For RO, the most used HD times in the reviewed studies were 3 and 4 hours (35.0%), followed by 1.5; 2 and 3.5 hours (10.0%). For OV, the times varied between 1 and 5 hours, with 3 hours being the most used time (56.0%), followed by 2 hours (18.0%), 4 hours (12.0%), 1 hour (6.0%), 5 hours (4.0%), 1.5 hours, and 2.26 hours (2.0%). Regarding EU, it has been determined that in 31 studies, the most considered HD time is 3 hours (64.5%), followed by 4 hours (22.6%), 2 hours (9.7%), and 3.5 hours (3.2%).
4. Hydrodistillation extraction yield
The percentage yield of the HD extraction process for the four plant species can be observed in Table 3. For MC, the yield varied between 0.06% and 2.00%, with the range of 0.50%-0.82% being the most reported (50.0%), followed by 0.06%-0.30% (35.0%) and 1.50%-2.00% (15.0%). It is appreciated that the Chamomile flowers EO can have a yield between 0.4% and 1.5%, depending on the habitat conditions of the plants (Bagheri et al., 2020; El-Hefny et al., 2019). Theoretically, it is known that chamomile (Matricaria spp.) flowers should contain a minimum of 4 ml/kg of essential oil (De Cicco et al., 2023); however, this value should be experimentally validated depending on the extraction and cultivation conditions of the plant.
For RO, the percentage yields obtained in the studies ranged between 0.35% and 3.53%, with the ranges of 2.50%-3.53% and 1.30%-1.86% being the most frequent (35.7% in both cases), followed by the range of 0.35%-0.72% with a lower number of studies (28.6%). These values, in addition to those already mentioned above, may be related to the rosemary drying method, the extraction technique, and the anatomical part of the plant used for extraction (Gourich et al., 2022). In the case of OV, the reported yield percentages ranged between 0.09% and 9.50%. The most frequently reported values were in the range of 2.00%-3.90% (24.42%), followed by 1.00%-1.75% (17.76%), 0.50%-0.90% (15.54%), 0.09%-0.45% (11.1%), 4.10%-5.00% and 5.30%-9.50% (8.88% in both cases). These variations may be due to a great diversity of factors such as soil conditions, harvest time, geographical location, and climatic and growing conditions (Brondani et al., 2018). For EU, the yield shown from 23 plant samples in the included studies ranged between 0.17% to 4.6%, with the range of 1.00%-1.82% being the most frequent (34.8%), followed by the range of 0.17%-0.96% (26.1%), 2.10%-2.90% (21.7%), and 3.10% -4.60% (17.4%).
5. Chemical profile of essential oils
In the chemical identification of extracted essential oils, the most common method is gas chromatography-mass spectrometry (GC-MS) (Lo et al., 2020); it is suitable for volatile samples that can evaporate upon heating (Gourich et al., 2022). Gas chromatography can separate analyte components by partitioning between the gaseous mobile phase and the stationary phase at different retention times; finally, all components are eluted, and the detector identifies them. The detector is a mass spectrometer that can decompose molecules into ionized fragments and detect these fragments at their characteristic mass-to-charge ratio (m/z) (Lo et al., 2020).
Table 3 shows the number of metabolites identified in each of the four plant species. For MC, the most frequent range was 31 to 52 compounds (34.8%), followed by 5 to 15 compounds (30.4%), and 17 to 24 compounds (26.1%). In some cases, a higher number of compounds, ranging from 70 to 75 (8.7%), were identified, consistent with reports on the phytochemical composition of MC essential oils, which have identified more than 120 compounds (De Cicco et al., 2023).
In studies of RO essential oils, the number of identified compounds varied from 9 to 66. The most common range was 9 to 18 compounds (40.0%), followed by 22 to 29 compounds (30.0%). Additionally, ranges of 31 to 33 compounds and 45 to 66 compounds were observed, each accounting for 15.0% of cases. The number and nature of these compounds are influenced to a greater or lesser extent by the thermal and hydrolytic effects during the HD process of RO essential oil (Filly, 2014). Regarding OV, the most frequently identified range of compounds was 3 to 19 (34.6%), followed by 20 to 39 compounds (30.7%), and 42 to 54 compounds (21.2%). Additionally, ranges of 64 to 77 and 79 to 158 compounds were identified, each accounting for 13.5% of cases. The analysis of 34 studies utilizing various species of Eucalyptus spp. revealed that EOs contained between 5 to 106 compounds. The most common range was 5-16 compounds (32.4%); then 17-28 compounds (29.4%); 32-48 compounds (26.4%); and 75-106 compounds (11.8%).
5.1. Chemical constituents of essential oils
The chemical constituents of EOs are mainly secondary metabolites, whose content and profile largely depend on various factors such as the stage of development, pedoclimatic conditions, drying, storage, and type of plant materials used (Heni et al., 2021). Generally, the primary components of EOs consist of monoterpenes and sesquiterpenes, which typically exist in the form of hydrocarbons or oxygenated compounds (Gladikostić et al. 2023). Monoterpenes are highly lipophilic compounds that interact with biological membrane constituents, altering their densities, fluidity, and physical arrangement of membrane phospholipids (Moghrovyan et al., 2019).
Table 2 Extraction conditions for essential oils by hydrodistillation in studies of plant species M. chamomilla, O. vulgare, R. officinalis, and Eucalyptus spp.
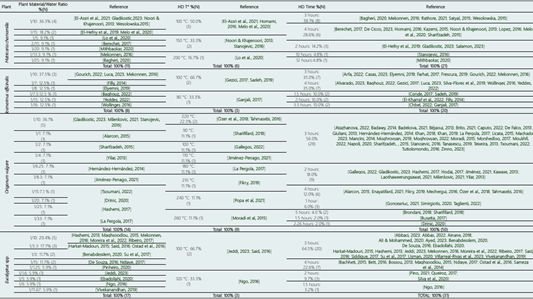
Note: %(N) = Percentage% (Number of studies); HD: Hydrodistillation; T°: Temperature.Table 3 presents the primary metabolites identified in the studies reported for the essential oils (EOs) of the four plant species. In the EO of M. chamomilla, the most frequently identified main metabolites were β-farnesene and α-bisabololol oxide A (30.5% for both metabolites), α-bisabololol oxide B (21.8%), α-pinene; bisabolone oxide; chamazulene and camphor (4.3% for each of the four metabolites).
Figure 3-A illustrates the main metabolites reported in the included studies. Chamazulene is a molecule formed from matricin, naturally present in the flowers during HD or steam distillation; the color of the oil determines its quality, as the blue color is due to the sesquiterpene. The most characteristic metabolites of chamomile EO reported in a previous study are terpenoids, with the most important compounds being bisabolol and its oxides A and B, farnesene, spatulenol, spiroethers, and azulenes, such as chamazulene (De Cicco et al., 2023). It is noteworthy that the oil extracted from chamomile flowers consists mainly of sesquiterpene derivatives (75%-90%), with only traces of monoterpenes (Singh et al., 2011). The chamazulene content of different chamomiles depends on the origin and age of the material, decreasing the longer the storage time of the flowers (Singh et al., 2011; EL-Hefny et al., 2019).
In the EO of RO, the most frequently identified major metabolites were 1,8-cineole (52.4%), camphor (23.8%), α-Pinene (19.0%), and limonene (4.8%). Oxygenated compounds present in the EO of R. officinalis (such as 1,8-cineole and camphor) tend to be more odorous than monoterpene hydrocarbons (Figure 3-B). Therefore, essential oils containing significant amounts of oxygenated compounds and less monoterpene hydrocarbons (for example, α-pinene and limonene) are more valuable (Yeddes et al., 2021; Filly, 2014). RO essential oil is known to exhibit high chemical variability, which may be related to its geographical origin, environmental conditions, harvest time or extraction method. Currently, chemotypes of RO essential oil are classified based on their major component (Casas et al., 2023).
For OV, the main identified metabolites were carvacrol (50.9%), thymol (18.9%), 4-terpineol (9.4%), germacrene D (5.7%), β-caryophyllene epoxide (3.7%), α-Pinene, γ-terpinene, eugenol, p-cymene, pulegone and trans-anetol (1.9% for each of the five metabolites) (Figure 3-0). Carvacrol is responsible for various biological activities of this plant, including antitumor, antimutagenic, antigenotoxic, antiparasitic, inhibition of acetylcholinesterase enzyme, antielastase, insecticidal, antihepatotoxic, and hepatoprotective activities. It is also used as a food additive, in bee breeding, and for the treatment of gastrointestinal conditions (Béjaoui et al., 2013). The high content of phenolic compounds, such as carvacrol and thymol, is present during the flowering stage of the plant. During this stage, the precursors of these phenolic compounds undergo a decrease in their content of p-cymene and γ-terpinene. In contrast, during the vegetative development stages, the amount of the phenolic portion decreases, while the amount of its precursors increases (Béjaoui et al., 2013).
In relation to EU, the most important active principles extracted are 1,8-cineole (63.9%), Citronellal, p-cymene and α-phellandrene (8.3% in all three cases). The remaining compounds are represented by camphene, estragole, globulol and limonene (2.8% in all this cases) (Figure 3-D). Notably, the presence of globulol in fruits of E. globulus has also been determined (Said et al., 2016), and estragole was identified in the same plant in a study where a mixture of leaves, stems, flowers, and other aerial parts was used in HD (Ainane et al., 2018).
5.2. Yield and chemical composition of essential oils according to plant material and extraction conditions:
Table 4 presents the yield and main metabolites obtained from the extraction of essential oils of MC, RO, OV, and EU, categorized by the origin of the plant species, type of plant material, part of the plant used, plant material/water ratio, temperature, and Hydrodistillation time. For MC, the highest yield percentages are observed for plant materials were 0.60-2.00%. The primary molecules commonly found in these extracts are β-Farnesene, α-Bisabolol oxide A, and α-Bisabolol oxide B.
Drying methods, temperature, and duration significantly impact essential oil production. Achieving low moisture content is crucial for stable matrices, inhibiting or limiting microbial activity in treated products. A study suggests that a final moisture content of <10% (wet basis) effectively restricts microbiological activity and could serve as an intermediate treatment to enhance subsequent operations such as extraction or mechanical crushing (Caputo et al., 2022). Depending on the type of plant material, it is observed that in dried samples of MC, the yield is higher (up to 2.00%) versus fresh material (up to 1.50%), as well as the diversity of main molecules present in dried material (α-Bisabolol oxide A; α-Bisabolol oxide B; Chamazulene; β-Farnesene and Bisabolone oxide) versus fresh material (α-Bisabolol oxide A and β-Farnesene).
Table 3 Yield and metabolites obtained in studies of essential oil extraction by hydrodistillation (HD) for plant species M. chamomilla, O. vulgare, R. officinalis, and Eucalyptus spp.

Note: %(N)= Percentage% (Number of studies); DS: dried sample.
These results are related to a study conducted in Egypt, where they compared the chemical composition of EO obtained from fresh and dried flowers of MC, using different techniques (sunlight, shade, oven, solar dryer, and microwave). The results showed that the main component of all EOs was α-bisabolol A oxide (33-50.5%); however, the drying methods used in this study significantly influenced the number of compounds identified, with 21 compounds after solar drying and only 13 after microwave drying (Abbas et al., 2022).
The sensitivity of volatile oils determines the appropriate temperature for drying processes; thus, higher temperatures promote the loss of more volatile components and the degradation of less stable substances (Ozdemir et al., 2018). In addition, air temperature influences both the quantity and quality of essential oils in aromatic plants, not only during drying, but also during storage (Moreira et al., 2022). Regarding the chemical composition as a function of the type of drying, it is known that sesquiterpene hydrocarbons predominate in fresh samples; shade-dried plant material contains many oxygenated monoterpenes and oxygenated sesquiterpenes (Bhatt et al., 2019).
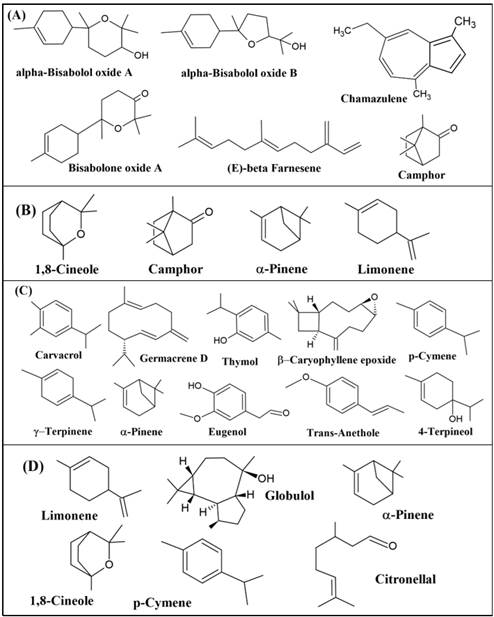
Figure 3 Molecules found in higher concentration during GC-MS chemical profiles of essential oils from Matricaria chamomilla (A), Rosmarinus officinalis (B), Origanum vulgare (C) and Eucalyptus spp. (D). Chemical structures created with ACD/ChemSetch Freeware 2020.2.1
Table 4 Yield percentage and metabolites obtained from essential oils by Hydrodistillation according to the origin, characteristics of the material used and conditions for the extraction of essential oils from plant species M. chamomilla; O vulgare; R. officinalis and Eucalyptus spp.
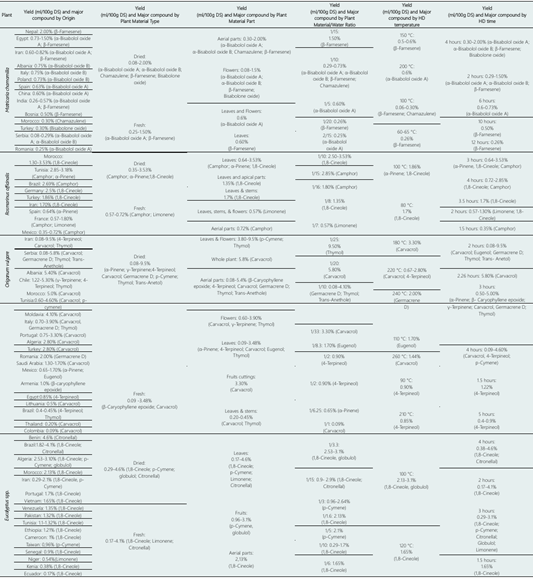
Note: HD: Hydrodistillation; DS: Dry Sample.
Regarding the part of the plant used for HD, aerial parts showed a higher yield of M. chamomilla EO with a yield of up to 2.00% and 4 major molecules identified, while in flowers the yield was up to 1. 50% and 4 molecules were identified; however, these values are higher than those found for yields of leaves alone and a mixture of leaves and flowers (0.6% in both cases), also reducing the variability of major compounds (β-farnesene and α-bisabolol oxido A). Reportedly, the quality of chamomile EO is generally determined by its blue color, which indicates a good amount of terpenoids (Piri et al., 2019). This is dependent on factors such as plant origin, drying techniques, extraction techniques, use of biofertilizers, among other factors (De Cicco et al., 2023; El Mihyaoui et al., 2022). The plant material/water ratio that yielded the highest EO extraction from MC was 1/15 (1.50%) with β-farnesene as the main metabolite; however, it is worth highlighting that the 1/10 ratio had a lower yield (0.29-0.73%) but a higher diversity of main metabolites (α-bisabolol oxide A; α-bisabolol oxide B; β-farnesene and chamazulene).
The HD temperature that produces the highest yield is 150 °C (0.5-0.6%) with one major metabolite (β-Farnesene), while reducing the temperature to 100 °C reduces the yield (up to 0.30%) but increases the variability of major compounds (β-Farnesene and Chamazulene).
With temperatures below 100 °C, the yield remains low (0.26%), and the variability of major metabolites (β-Farnesene) is also reduced. The HD extraction time of MC essential oils for 4 hours showed the best yield (0.30-2.00%) and the highest variability of major metabolites (α-Bisabolol oxide A; α-Bisabolol oxide B; β-Farnesene; and Bisabolone oxide). It is worth noting that a 2-hour HD produces a lower yield (0.29-1.50%); however, the variability of its major metabolites is like the 4-hour HD time (α-Bisabolol oxide A; α-Bisabolol oxide B and β-Farnesene). Increasing the distillation time to between 6 to 12 hours reduces the percentage yield to 0.73% and 0.26%, respectively, and in both cases, the major metabolite identified is β-Farnesene.
The extraction yields of Rosmarinus officinalis were highest in Morocco and Tunisia (around 3.5%). The main compounds found were 1,8-Cineole, α-Pinene, Camphor, and Limonene. The yield of dry material was higher than that of fresh material (3.52% and 0.72%, respectively). The leaves of RO had the highest yield (up to 3.53%) with metabolites identified as Camphor, α-Pinene, and 1,8-Cineole. It is important to note that Limonene was observed as the most abundant compound when flowers were added as a sample. The highest yield for RO was achieved with a plant material to water ratio of 1:10, resulting in up to 3.53% of essential oil extracted. In this ratio, 1,8-Cineole was identified as the most abundant metabolite. Additionally, a high yield of 2.85% was achieved with a 1:15 ratio, with Camphor being identified as the most abundant compound. The studies reported HD temperatures of 80 °C and 100 °C, which resulted in close yields of 1.70% and 1.86%, respectively. The compounds obtained were 1,8-Cineole at both temperatures and α-Pinene at 100 °C. The highest yield of 3.53% and the highest diversity of metabolites, including α-Pinene, 1,8-Cineole, and Camphor, were obtained after 3 hours of HD time. However, it was observed that the yield was low (0.35%), and the major metabolite produced in a shorter HD time (1.5 hours) was Camphor, in comparison to the other times.
For O. vulgare, the highest yields were 5.00-9.5%, with the main metabolites most frequently found being carvacrol, 4-terpineol, and thymol. When considering the type of plant material used, dried samples obtained a higher yield (up to 9.5%) compared to fresh material (up to 3.48%). Dried material also yielded a greater number of main compounds (carvacrol, thymol, α-pinene, p-cymene, germacrene D, 4-terpineol, γ-terpinene, trans-anethole, pulegone, and eugenol) compared to fresh material (carvacrol and β-caryophyllene epoxide). In a study on the influence of drying on the extraction of EO from OV, the highest yield of essential oil was obtained with sun-dried material (0.30% w/w), while the material dried in the shade and oven at 35 °C and 45 °C had a yield of 0.20%. This effect on oil production is due to the modification of the biological structure of the oil glands after the rupture of the trichomes (Bhatt et al., 2019).
Aerial parts from OV obtained a higher yield (up to 5.4%) than other parts used, and they also presented the highest diversity of main metabolites (carvacrol, pulegone, 4-terpineol, trans-anethole, germacrene D, β-caryophyllene epoxide, and thymol). However, extraction using only O. vulgare leaves resulted in a lower yield (up to 3.48%) but presented an interesting number of major compounds (carvacrol, thymol, α-pinene, germacrene D, 4-terpineol, and eugenol).
The plant material/water ratio used in HD with the best results in yield was 1/25 (9.50%) with thymol as the main metabolite, while a ratio of 1/20 reported carvacrol as the main metabolite. However, the plant material/water ratio of 1/10, although reporting a lower yield (up to 4.10%), showed a higher number of main metabolites (germacrene D and thymol). In the other ratios analyzed in this review, the yield was lower than 3.30%, with carvacrol being the main metabolite in most cases.
The HD temperature with the best results in yield for OV was 180 °C (up to 3.30%), with carvacrol as its main metabolite. At 220 °C, the yield decreased to up to 2.80%, but a greater number of main metabolites was identified (carvacrol and 4-terpineol). Temperatures higher than 220 °C resulted in a low yield (up to 2.00%), with carvacrol and 4-terpineol remaining as the main molecules. However, temperatures below 180 °C yielded up to 1.70%, with the identification of carvacrol, eugenol, and 4-terpineol as main metabolites at low temperatures.
The HD time for O. vulgare with the highest yield is 2 hours (up to 9.5%), identifying major metabolites such as carvacrol, thymol, germacrene D, trans-anethole, and eugenol. Meanwhile, a HD time of 3 hours has a lower yield (up to 5.00%) but a higher number of major metabolites identified (carvacrol, thymol, α-pinene, germacrene D, γ-terpinene, and pulegone). As the HD time increases above 3 hours, the yield is still low (4.60%), and the identifiable major metabolites are reduced to carvacrol, 4-terpineol, and p-cymene.
For Eucalyptus sp. the highest yields have been seen in plant material were 3.1% - 4.6%. 1,8-Cineole has been found to be a major compound in research studies that mention its yield. Similar yields (ranging from 1.32% to 2.13%) In all cases, the main metabolite found was 1,8-Cineole. The material from Algeria was more diverse in chemical composition, with 1,8-Cineole, p-Cymene, and globulol reported as the main metabolites. There was no significant difference in yield between fresh and dry materials (4.1% and 4.6% respectively). However, differences were observed in the chemical compounds obtained. Both materials contained 1,8-Cineole and Citronellal, but dry samples also contained p-Cymene and globulol, while fresh samples contained Limonene.
In terms of yield, leaves were the plant part with the highest yield in HD (up to 4.6%) and with the highest diversity of metabolites (1,8-cineole; p-cymene; limonene and Citronellal), while fruits and aerial parts had lower yields (3.1% and 2.13%, respectively) and also reduced the number of main metabolites found (p-cymene, globulol in fruits and 1,8-cineole in aerial parts). The plant material/water ratio that resulted in the highest yield was 1/3.3 (3.1%). Ratios of 1/15 (up to 2.9%) and 1/3 (up to 2.64%) also produced significant yields. However, the first two ratios produced a greater diversity of main metabolites, including Globulol and Citronellal, respectively, in addition to 1,8-Cineole. The highest reported HD times were 100 °C and 120 °C, with decreasing yields at higher temperatures (3.1% and up to 1.65%, respectively). Lower temperatures resulted in a higher number of identified metabolites. The optimal time for higher yields and major metabolites (1,8-Cineole, p-Cymene, Citronellal, Globulol, and Limonene) was found to be 3 hours, with HD times ranging from 2 to 4 hours (3.1% - 4.6%). It is important to note that the yield and chemical composition of Eucalyptus EO can vary depending on factors such as the species, leaf maturation during collection, sample preparation and the season in which it was obtained (Usman et al., 2020).
6. Current and future challenges
The extraction of essential oils from aromatic plants presents numerous challenges, including small extraction yields, high plant material costs, variable chemical compositions of the oils, and the generation of solvents and plant waste. In the search for sustainable and efficient extraction, modern methods have gained prominence, including microwave-assisted, supercritical and ultrasonic extraction techniques. These innovative approaches have circumvented the inherent limitations of conventional methods and provide new opportunities to harness the full potential of essential oils (Olalere et al., 2024).
The increasing demand in the global supply chain for essential oils faces the challenge of maintaining consistent quality standards, which is why standardization and optimization of the extraction process is crucial to address these challenges (Smith, 2024).
Quality and yield are influenced by various factors such as geographical region, climate, plant varieties, genetics, drying techniques, and extraction conditions (e.g., plant material-to-water ratio, extraction temperature, and time). Future challenges for the study of essential oil extraction processes are directed towards the use of nanotechnologies to ensure the least possible degradation and access to the sites of chemical activity of essential oils as nanocapsules, nanoemulsions and nanofiber membranes (Zhang et al., 2024). Despite advances, optimizing extraction conditions, encapsulation and ensuring the stability of EOs remain key challenges. Ongoing research promises innovative solutions to maximize the therapeutic potential of essential oils (Rodilla et al., 2024).
7. Conclusions
Optimization of extraction conditions as well as proper conditioning of plant material are essential to obtain higher volumes of essential oils at lower cost and ensure better quality, adding value to the product. Although new extraction methods exist, hydrodistillation remains the most common and cost-effective. Therefore, understanding the optimal extraction conditions is critical to maximize the production and quality of essential oils.