Introduction
Poor microbiological air quality can affect the health status of hospitalized patients in critical areas, increasing the risk of nosocomial infections1 as well as health workers2. Different studies have shown colony-forming unit (CFU) counts higher than the limits established for mesophilic microorganisms3,4. The most frequently isolated phenotypes are gram-positive cocci (51% Staphylococcus and 37% Micrococcus) and fungi (41% Penicillium and 24% Aspergillus)3. Added to this problem is the high proportion of antibiotic resistance in isolated microorganisms5. In fact, with the current COVID-19 pandemic, it has been shown that 7.1% of hospitalized patients had nosocomial infection, with the most frequently isolated microorganisms being coagulase-negative Staphylococcus (27.9%), Acinetobacter sp. (20.9%) and Pseudomonas aeruginosa (14.0%), also with almost 4 times higher chance of mortality than in COVID-19 patients without nosocomial infection(6).
Ozone (O3) is a colorless gas produced by the action of ultraviolet rays and has electrophilic and oxidative properties7. Due to this, it is used as an effective antibacterial agent and as a disinfectant for multiple microorganisms8,9 including airborne viruses10. The use of portable systems to generate ozone is widely used in the control of microorganisms on surfaces and air in hospital environments11, and even its effectiveness has been demonstrated in the COVID-19 context12. Various studies have shown that ozone concentrations between 50 and 500
µg/m3 for 1 hour significantly reduced the CFU count, especially gram-positive microorganisms13,14. However, prolonged exposure to ozone can cause adverse health effects in exposed patients and workers15, so it is necessary to assess new protocols for ozonation systems in shorter periods for the control of microorganisms in environments.
Hospital conditions regarding microbiological air quality in Peru are not ideal. Various studies have shown that overcrowding and inadequate ventilation are associated with an increase in nosocomial infections16. This has a direct impact on critical areas such as surgical centers17, promoting an increase in the hospital stay of the patients treated. Added to this, in Peru, there are no parameters or reference levels for microorganisms in air in health environments or reports in this regard18. Consequently, our study aims to evaluate an Ozone Generating System (OGS) through tests to identifying evaluate the control of microorganisms in the air of the sanitary environments of the National Institute of Health (INS) from Peru.
The study
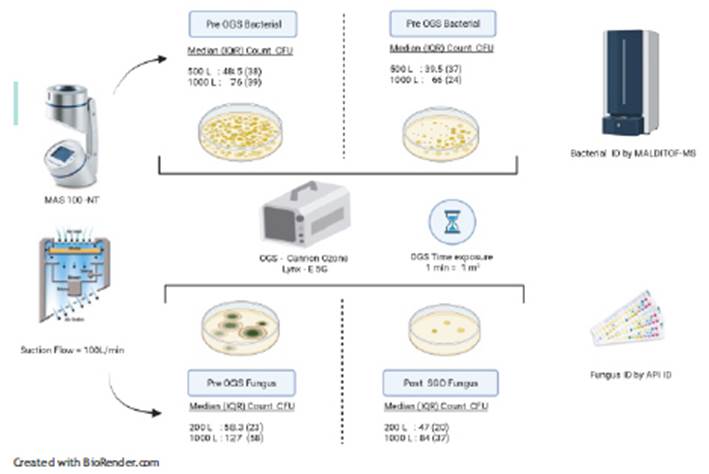
Prior to the study, we performed a standardization of the sampled air volume in order to obtain a representative CFU count between 30 and 300, obtaining as appropriate volumes between 500 and 1000 L for bacteria and between 200 and 1000 L for fungi. These conditions allow for a uniform colony count without overcrowding19. Before the exposure with OGS, the windows and doors of the health environments were closed and the time established to achieve a concentration >5 ppm of ozone according to the footage of the environment was taken.
For samples was considered the total number of health environments (ten) where medical care was provided at the INS, this selection was not probabilistic. The OGS used was the Lynx-E 5G portable cannon (Water & Energy Solutions)3 with a production capacity of 5000 mg of ozone per hour. Each sanitary environment was exposed to the OGS for a time of 1 min per m3 We performed pre and post-exposure air sampling to OGS by the impaction method using the multi-hole Microbiological Air Sampler (MAS-100 NT, Merck) programmed at a suction flow rate of 100 L/min. Temperature and humidity conditions were determined using a thermohygrometer (Traceable). Plate Count Agar (PCA) culture media were used, incubated at 35°C±2°C for 48 hours for the total count of bacteria CFU; and Oxytetracycline Glucose Agar (OGA), incubated at 25°C±2°C for 5 days for total fungi CFU count.(20) After standardization, volumes of 200 L, 500 L, and 1000 L of air from each health environment were sampled. Bacteria were identified by mass spectrometry (MALDITOF, Bruker) and fungi, by biochemical tests (API ID, Biomerieux) (Figure 1).
The CFU count of bacteria and fungi before and after exposure to OGS was presented in terms of CFU/m3 according to the dimensions of each health environment and they were summarized with median measurements that represent the set of health environments. The median CFU/m3 for fungi and bacteria obtained before and after OGS were compared by Wilcoxon's non-parametric rank sum test according to the volumes of air sampled.
Findings
The sanitary environments were mostly medical offices that were adjacent to gardens and water sources for medical consultations. The temperature and relative humidity range was 17-26°C, and 58-87% respectively.
We determined the CFU/m3 obtained for each of the 10 sanitary environments evaluated, grouped by volume of air sampled (Table 1). Regarding the bacterial CFU count, the median CFU/m3 obtained with a sampled air volume of 500L pre and post exposure to OGS was 48.5 (IQR: 38) and 39.5 (IQR: 37), respectively; not showing significant difference (p=0.241). Likewise, the median CFU/m3 obtained with a sampled air volume of 1000L before and after exposure to OGS was 76 (IQR: 39) and 66 (IQR: 24), respectively; not showing significant differences (p=0.066). Regarding the fungal CFU count, the median CFU/m3 with an air volume of 200L was 58.2 (IQR: 23) and 47 (IQR: 20), before and after exposure to OGS, respectively, being statistically significant (p=0.047). In addition, with a sampled air volume of 1000L, the median CFU/m3 in fungi was 127 (IQR: 58) and 84 (IQR: 37) before and after exposure to OGS, respectively; this difference being statistically significant (p<0.005) (Figure 2). Bacterial typing, using mass spectrometry, identified 17 species, while fungal typing using biochemical tests identified 35 species (Table 2). Among the fungi, species of Aspergillus spp. (12/35: 34.3%), Penicillium spp. (8/35: 22.9%), Neoscytalidium dimidiatum (8/35: 22.9%), Hyalohyphomycete (2/ 35: 5. 7%), Gliocladium spp. (2/35:5.7%), Cladosporium herbarum (1/35: 2.9%), Scedosporiums apiospermum (1/35: 2.9%) and Acremonium spp. (1/35: 2.9%). Bacterial typing included species of Pseudomona oryzihabitans (3/17: 17.6%), Pseudomona stutzery (3/17: 17.6%) and Pantoea agglomerans (2/17: 11.8%) and unidentified bacterial species (9/17: 52.9 %).
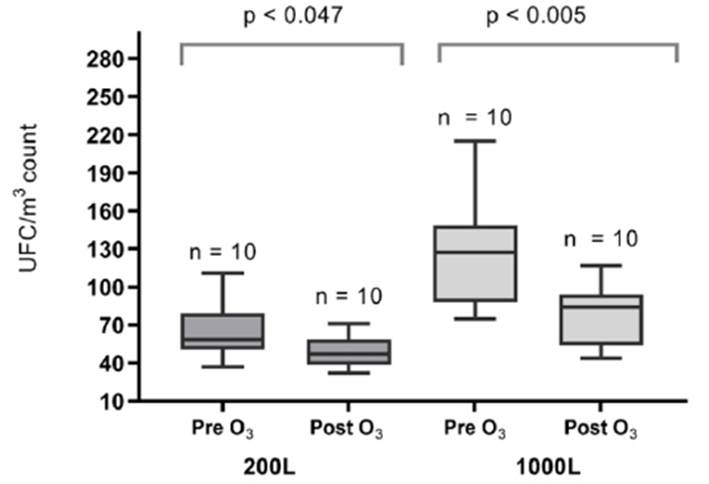
Figura 2 Distribution of the CFU/m3 count of fungi isolated in INS environments before and after exposure to OGS.
Tabla 1 Distribution of the CFU/m3 count of bacteria and fungi in 10 sanitary environments of INS.
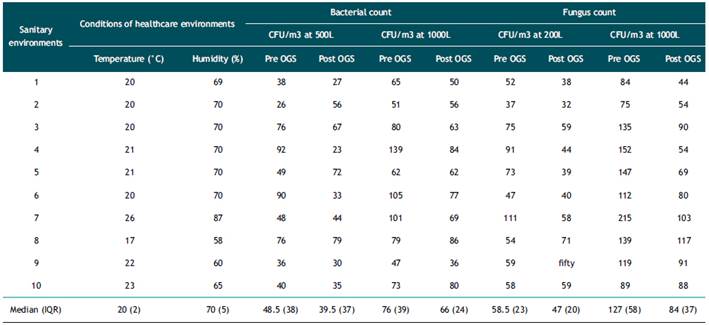
IQR: interquartile range
Discussion
Our study evaluated the biocidal activity of ozone for the control of microorganisms in air samples from health environments based on its oxidative properties21-23. Main result shows a significant reduction in the median fungal CFU/m3 count for the set of health environments evaluated at a standardized air sampling volume of 200L and 1000L. However, we did not find a significant reduction in the concentration of environmental bacteria. Similar results were obtained by Pastuszka et al. using the EWG (electron wind generator) methodology, in which the reduction of CFU/m3 levels in both bacteria and fungi was evaluated, reaching a reduction of up to 80%24. Likewise, a study based on the generation of ozone obtained a significant reduction of environmental microorganisms in administrative and surgical areas11. Our study achieved a reduction of 66.4% (84/127) of CFU/m3 for fungi in a sampling volume of 1000L. According to parameters of the Directive for the measurement of microorganisms and endotoxins in suspension in the air (UNE 171330:2)25, the levels found are within the permitted limits.
The bacteria frequently identified in the study were gram- negative bacilli of the genus Pseudomonas oryzhabitans (also called Flavimonas oryzihabitans), Pseudomonas stutzery and Pantoea agglomerans. These bacteria are commonly found in soils, humid environments and diverse ecosystems, being able to adapt to hostile environments and generate opportunistic infections26,27. Pseudomonas oryzihabitans, belongs to the subgroup of non-fermenting bacilli Ve-2, linked to central nervous system infections, catheter- associated bacteremia, among others28. For its part, Pseudomonas stutzery is widely distributed in the environment with a relatively low virulence. However, since 1950, its isolation from human biological samples such as blood, pus, skin, synovial tissue, joint fluid and cerebrospinal fluid has been reported29. Pantoea aglomerans is a beneficial bacterial for plants, it promotes plant growth, a property that gives it biotechnological properties, its main use being fungal control30. However, it occasionally produces outbreaks of nosocomial bacteremia31.
The fungi were inhibited in their growth after the use of OGS, however, species of Aspergillus spp, Penicilium spp. And Neosytalidium dimidiatum grew after exposure to OGS. The species of Aspergillus spp. is associated with the occupational disease called allergic bronchopulmonary aspergillosis (ABPA)32. For its part, species of Penicillium spp.grew up in environments where there is an accumulation of documentation, this fungus has biodeteriorating properties and is a producer of hemolysins that are pathogenic for humans 33. Neoscytalidium dimidiatum causes onychomycosis and scytalidiosis, and has been described as the agent responsible for causing endophthalmitis, sinusitis, osteomyelitis, mycetomas, invasive cerebral phaeohyphomycosis, and disseminated infections in immunosuppressed patients34. The diversity and greater frequency of fungi found in the evaluated environments may be due to the presence of abundant documentary material such as medical records, records, care sheets, among others, and the proximity to gardens. Although the medical care provided in health environments is not focused on immunocompromised patients, it is necessary to establish complementary measures to control environmental microorganisms.
In conclusion, the OGS does not result in a statistically significant reduction in bacteria count, but it does inhibit the growth of most fungi in the health environments. Our results show that the OGS is an effective method for the decontamination of environmental fungi. However, it is necessary to develop new studies to generate consistency on the effectiveness of OGS in fungi and bacteria decontamination in health environments, especially in critical hospital areas.