Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.2 Lima abr-jun 2020
http://dx.doi.org/10.31403/rpgo.v66i2251
Case Report
Management of eutocic delivery in a patient with COVID-19 in Lima, Peru
1 Delgado Clinic - AUNA, Lima, Peru
2 Cayetano Heredia University, Lima, Peru
We present the case of a eutocic, uncomplicated delivery in a patient positive for COVID-19. The patient, a 33-year-old woman, 39 weeks pregnant, who had received prenatal care in a private clinic, presented in labor, coughing, without any other symptoms. She was diagnosed with COVID-19 by rapid test, IgM (+) and IgG (-). We isolated the patient and provided personal protective equipment following our clinic’s protocol. Delivery was managed according to obstetric conditions, applying epidural anesthesia in the active phase; the baby was born without complications. Nor skin-to-skin contact nor delayed umbilical cord clamping were performed. Mother and child were discharged without complications after the newborn completed the required isolation period, testing negative for COVID-19. Telephone follow-up was performed. The healthcare team followed the recommended protocol to manage delivery during the COVID-19 pandemic.
Key words: Coronavirus infections; COVID-19; SARS-CoV-2; Obstetric Delivery; Birth settings; Delivery rooms
Introduction
In December 2019, in Wuhan, China, a virus of the Coronaviridae family that had not been described in humans yet was identified as the cause of a new form of severe acute respiratory syndrome, and named SARSCoV-2. The disease it causes would afterwards be known as COVID-191.
As of 4 May 2020, there have been 43 372 confirmed cases of COVID-19 in Peru2. In this context, there is a large number of pregnant women who will get infected with SARS-CoV-2, regardless of the severity of symptoms they will develop. Because of this, while the pathogen’s behavior remains under study, it is important to show the current experience in managing delivery in infected women.
This case report describes the labor, delivery and puerperium in a patient positive for COVID-19 without complications; the outcomes of mother and newborn were favorable, as well as those of the healthcare team, who followed adequate protective measures.
Case report
On 13 April, at 10.00 hours, a 33-year-old woman, gravida 4, para 2, abortus 1, was admitted to the emergency room. Her prenatal controls had taken place in our clinic, without any complications.
The patient reported a history of 4 weeks of dry cough, without fever nor any other symptoms.
In the Labor-delivery-recovery room (LDR), the patient and her husband were both protected with a surgical mask. She was tested for COVID-19 with the rapid blood test (Cellex SARS-CoV-2 IgG/IgM Rapid Test) and the molecular test (RT-PCR SARS-CoV-2), which used a nasopharyngeal swab sample.
At 13.00 hours, the result of the rapid test was obtained: IgM (+), IgG (-). Patient and partner were informed that, following the established protocol, the presence of an accompanying person was not allowed in this case. The patient, wearing an N95 mask, was transferred to an LDR room especially prepared for isolating patients with COVID-19 (Figure 1), and health workers were provided with class 3 personal protective equipment (PPE) (Figure 2).
Patient’s vital signs on admission were: 90 heartbeats per minute, blood pressure 100/52 mmHg, respiratory rate 20 rpm, temperature 36.5°C, oxygen saturation 99%. We performed all the recommended laboratory tests, which showed no significant alterations (Table 1). Maternal functions were stable. Fetal monitoring was continuous and stayed in category I, with a baseline of 145 beats per minute, moderate variability, no decelerations, and uterine contractions every 3 minutes.
Table 1 Laboratory test results.
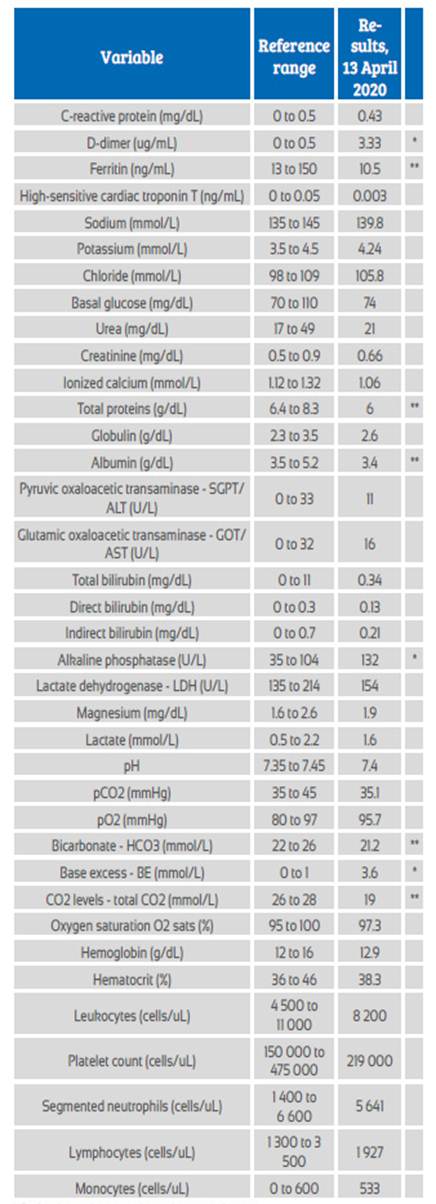
* Patient’s results were above the normal range
** Patient’s results were below the normal range
The attending anesthesiologist applied epidural analgesia in the LDR room upon patient’s request, when her cervix had dilated to 6 cm, following the precautions regarding the use of PPE.
Vaginal delivery occurred at 18.30 hours without any major complications; the female newborn weighted 3 020 g, with a length of 49 cm and an Apgar score of 9 at one and five minutes. The medical team did not perform episiotomy, delayed clamping nor skin-to-skin contact.
The baby was transferred to the room for healthy newborns. Samples were obtained for both types of COVID-19 tests and formula feeding and isolation from the mother were indicated.
In the postpartum, the mother had no symptoms and her obstetric outcome was satisfactory. She was discharged after 24 hours, with the following indications from an evaluation by the specialist in infectious diseases: no antiviral therapy, paracetamol 1g PO for sore throat, N-acetylcysteine 600 mg PO every 8 hours, contact tracing among her family, home isolation and education about alarm symptoms. Daily telephone follow-up confirmed an improvement of respiratory symptoms, without any other events.
One day after discharge, the molecular test was informed as negative.
The patient’s husband stayed at home following safety precautions. His results for the rapid and molecular tests after 48 hours were negative.
One week after delivery, the tests were repeated, with these results: IgM (+), IgG (-), molecular test negative. CT scan of the chest was informed as normal.
Similarly, rapid and molecular tests were applied to the husband for a second time and to their relatives, yielding negative results.
At day 8 after birth, the newborn was discharged, with negative results for both rapid and molecular tests. The indications were feeding expressed milk and following preventive measures until 14 days of isolation were completed.
Three weeks after delivery, mother and newborn have progressed favorably. Furthermore, the health workers who attended the delivery remained asymptomatic.
Discussion
This case report presents the management of vaginal delivery in a patient positive for COVID-19, diagnosed by rapid test and attended in a dedicated LDR room, with adequate PPE3,4.
The patient was admitted in labor and screened for COVID-19 with the rapid test; due to her symptoms, this was complemented with molecular testing. Given the large number of asymptomatic carriers, universal screening in newly admitted patients must be considered when the necessary implements are available5,6. This is why all patients attended for vaginal delivery or C-section undergo universal screening with both types of tests in our clinic. Obtaining both tests helps in the diagnosis of patients whose RT-PCR (reverse transcription polymerase chain reaction) molecular test appears negative due to an undetectable viral load, which occurs approximately 2 weeks after symptom onset. On the other hand, IgM and IgG antibodies peak at week 2 or 3 after symptom onset, and decline between weeks 5 and 77.
The patient was attended in an isolated LDR room prepared for cases with COVID-19, by health workers exclusively designated to provide delivery care, geared with adequate PPE, as recommended by current guidelines6.
The LDR room assigned for COVID-19 patients has the same area and equipment as the other five LDR rooms in the institution, which provides the necessary space for the adequate management of labor according to obstetric conditions, such as walking, exercise, feeding, wireless electronic fetal monitoring, bath, hot shower, family accompaniment, labor analgesia, and delivery, immediate puerperium and newborn care.
Management of the first stage of labor should not be altered, except for cases of maternal instability. It is not contraindicated to use oxytocin or amniorrhexis to accelerate labor and de-crease time of exposition6.
Epidural analgesia was applied as usual, not only for need, but also because it is recommended to provide it early if an emergency C-section was required. This type of surgery could use general anesthesia, which implies a risk for the health staff attending patients with COVID-196,8.
Despite the isolation, the husband accompanied the patient virtually throughout the entire delivery, a situation that is currently considered in guidelines for managing this scenario6.
It was decided to conduct a vaginal delivery be-cause infection by COVID-19 per se is not an indication for C-section. Mode of delivery has to be determined by obstetric indications6,9-11.
Neither skin-to-skin contact nor delayed umbilical cord clamping were performed. Given the unresolved concerns about vertical transmission, these practices are not recommended in patients under suspicion or positive for COVID-196.
Patient and newborn were isolated until discharge. Their contacts (husband and children) underwent rapid and molecular tests that resulted negative before newborn discharge. Contact tracing is recommended in all patients positive for COVID-1912.
Telephone follow-up was used to assess respiratory symptoms and for postpartum monitoring12,13.
Delivery care for COVID-19 positive patients, according to current recommendations, requires access to universal screening, an isolated and appropriately prepared LDR and the correct use of PPE.
REFERENCES
1. World Health Organization. Coronavirus Disease (COVID 19) outbreak. https://www.who.int [ Links ]
2. Ministerio de Salud. Sala Situacional Covid-19 Perú. https://covid19.minsa.gob.pe [ Links ]
3. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages: interim guidance, 6 April 2020. https://apps.who.int/iris/handle/10665/331498 [ Links ]
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for inpatient obstetric healthcare settings. https://www.cdc.gov/coronavirus/ 2019-ncov/hcp/inpatient-obstetric-healthcare-guidance. html [ Links ]
5. Vintzileos WS, Muscat J, Hoffmann E, Vo D, John NS, Vertichio R, Vintzileos AM. Screening all pregnant women admitted to Labor and Delivery for the virus responsible for COVID-19 [published online ahead of print, 2020 Apr 26]. Am J Obstet Gynecol. 2020;S0002-9378(20)30472-5. doi:10.1016/j.ajog.2020.04.024 [ Links ]
6. Boelig RC, Manuck T, Oliver EA, Di Mascio D, Saccone G, Bellussi F, Berghella V. Labor and delivery guidance for COVID- 19. Am J Obstet Gynecol MFM. 25 March 2020. https://doi.org/10.1016/j.ajogmf.2020.100110 [ Links ]
7. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. Published online May 06, 2020. doi:10.1001/jama.2020.8259 [ Links ]
8 8. The Society for Obstetric Anesthesia and Perinatology. Interim considerations for obstetric anesthesia care related to COVID19. March 23, 2020. https://soap.org/education/provider-education/expert-summaries/interim-considerations-for-obstetric-anesthesia-care-related-to-covid19/ [ Links ]
9. Chen D, Yang H, Cao Y, Cheng W, Duan T, Fan C, et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynecol Obstet. 2020;149(2):130-6. doi:10.1002/ijgo.13146 [ Links ]
10. Yang Z, Wang M, Zhu Z, Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med. 2020;1-4. doi:10.1080/14767058.2020.1759541 [ Links ]
11. Della Gatta AN, Rizzo R, Pilu G, Simonazzi G. COVID19 during pregnancy: a systematic review of reported cases [published online ahead of print, 2020 Apr 17]. Am J Obstet Gynecol. 2020;S0002-9378(20)30438-5. doi:10.1016/j.ajog.2020.04.013 [ Links ]
12. Royal College of Obstetricians & Gynaecologists. Coronavirus (COVID-19) infection and pregnancy. Information for healthcare professionals. Version 4. March 23, 2020. [ Links ]
13. Centers for Disease Control and Prevention. Coronavirus disease 2019 (CPVID-19). Pregnancy, breastfeeding, and caring for young children. https://www.cdc.gov/coronavirus/ 2019-ncov/need-extra-precautions/pregnancy-breastfeeding. html [ Links ]
Received: May 04, 2020; Accepted: May 08, 2020











 texto en
texto en