INTRODUCTION
Varroosis is a parasitic disease caused by Varroa destructor, an obligate ectoparasite of Apis mellifera, which feeds on the fatty body and hemolymph of adult bees, causing loss of body weight and malformations (Ramsey et al., 2019). Its presence facilitates the entry of other agents, such as viruses and bacteria, and significantly reduces honey production, by reducing the life span of the bees (DeGrandi-Hoffman et al., 2017; Gregorc et al., 2022). It is one of the six most relevant diseases in honeybees and the main health problem for beekeeping (DeGrandi-Hoffman et al., 2016; Jack & Ellis, 2021). In Chile, it has been reported since 1992, and it extends throughout the territory where honeybee colonies exist, as it has a cosmopolitan distribution (SAG, 2020; Lamas et al., 2023).
The economic repercussions associated with varroosis are relevant for a country like Chile, which depends on the pollination efficiency for fruit and vegetable production, which generates an income close to USD 6 billion per year. Additionally, beekeeping activities allow the exportation of 4474 to 10403 tons of natural honey, with an estimated value of 13-29 million dollars, placing Chile in the 29th place in the ranking of honey exporting countries, with Germany and France as the main destination markets (Trade Map, 2023).
To ensure pollination services in the country, some 920142 honeybee colonies are registered. Of them, around 45% are located in the central regions (SAG, 2020), where the highest percentage of fruit crop area is concentrated (ODEPA, 2020). The pollination efficiency and productive performance of honey bees depend on the number of bee colonies and their strength. Therefore, proper preparation in terms of health and nutrition is necessary to have competent honeybee colonies, keeping the level of infestation by Varroa destructor mites as low as possible and therefore having the least amount of impact on the bees and their output (Mortensen et al., 2023).
Varroosis prevalence and variations in the infestation rates in adult bees are attributable to multiple factors, including the environmental effect and genotype of the bee (Meixner et al., 2015), and management practices of the honeybee colonies (Giacobino et al., 2015). Although the threshold of parasitic infestation from which damage occurs in the colony has not been determined precisely, it has been reported that an acceptable infestation rate should not exceed 3% in adult bees, since higher rates generate significant productive losses, and decrease the bees’ survival (Giacobino et al., 2014).
To successfully mitigate the harmful effects of this parasitosis and to reduce the use of acaricides, in different countries, integrated management pro-grams have been reported that use epidemiological criteria to determine which factors increase para-site infestation rates in populations of honeybees (Verde et al., 2012; van Engelsdorp et al., 2013; Giacobino et al., 2014). A more comprehensive understanding of the epidemiology of varroosis would make it easier to establish an integrated management program, so the current work's goal is to investigate various epidemiological aspects of varroosis in honeybees from Chile's Central Region apiaries.
METHODOLOGY
Experimental design and data collection
To obtain information related to the epidemio-logical aspects of varroosis and to establish possible factors associated with this parasitosis, 58 apiaries located between 32º42' and 34º07' South latitude, and between 70º01' and 71º08' West longitude were studied, covering three regions of Central Chile (Valparaíso, Metropolitana and O’Higgins). Five field monitorings were carried out during the years 2015 and 2016, at different times of the year (autumn, spring, and summer), applying a qualitative survey to the beekeepers on each visit. In this survey, observations related to beekeeping management were recorded, such as the number of bee colonies per beekeeper, colony migration (transhumance) or pollination services, delivery of food supplements during periods of floral resources scarcity (energy food or protein), and the application of treatments against the control of Varroa sp. According to the number of bee colonies per beekeeper and the criteria established by ODEPA (2018), the surveyed beekeepers were classified as small (1 to 299 colonies), medium (300 to 799 colonies), large (800 to 1499 colonies) and very large (> 1500 colonies).
The first monitoring (M1), with 47 beekeepers, was carried out between April and May 2015 in the autumn season. The second monitoring (M2) was carried out between September and October 2015, during spring (pre-harvest), and included 32 beekeepers. The third monitoring (M3) was between December 2015 and January 2016, during harvest time (summer) and 26 beekeepers participated. Finally, monitoring 4 and 5 were carried out during the year 2016, between April and May (M4), and between September and October (M5), with the participation of 39 and 53 beekeepers, respectively. The apiaries and monitored bee colonies (Langstroth type) were selected at random and the beekeepers voluntarily joined the study.
Determination of the infestation rates by Varroa sp. (IRV%) in adult bees and their prevalence
The infestation rates by Varroa sp. (IRV%) in adult bees and their prevalence were determined using a standard method (Dietemann et al., 2013), taking approximately 300 adult bees from the periphery of the frames with closed brood. The samples were kept in sealed containers with clearly labeled lids that contained a 75% ethanol solution. A total of 591 samples of adult bees were processed, corres-ponding to three colonies for each apiary visited.
Honeybee conlony strength
The honeybee colony strength was determined through the semi-subjective Liebefeld method with modifications, based on visual estimates from an observer (Delaplane et al., 2013; Dainat et al., 2020). Briefly, brood chambers were inspected, and the number of comb sides with open and closed brood, adult bees, and food reserves (honey and pollen) was recorded, according to Olate-Olave et al. (2021). Additionally, to estimate the general situation of the colonies, and the flight behavior of the bees, the number of bees entering the hive in a period of one minute was recorded.
Statistical analysis
Data were processed, weighted, and entered according to the date of the monitoring and the type of variable. The software IBM SPSS 22.0 was used for all statistical and descriptive analyses. According to each variable, the descriptive analysis was expressed as a percentage (%) relative to the total number of beekeepers, as well as arithmetic mean, median, minimum, and maximum values. Additionally, tests of normality (Shapiro-Wilk, α = 95%) and homoscedasticity (Levene test, α = 95%) were applied to determine the distribution and homogeneity of the variances, respectively. Based on the results, non-parametric tests (Kruskal-Wallis o U de Mann-Whitney, α = 0.05) were performed to find possible differences in the infestation rates by Varroa sp. according to the different categorical variables.
RESULTS AND DISCUSSION
Characterization of the beekeepers according to the survey
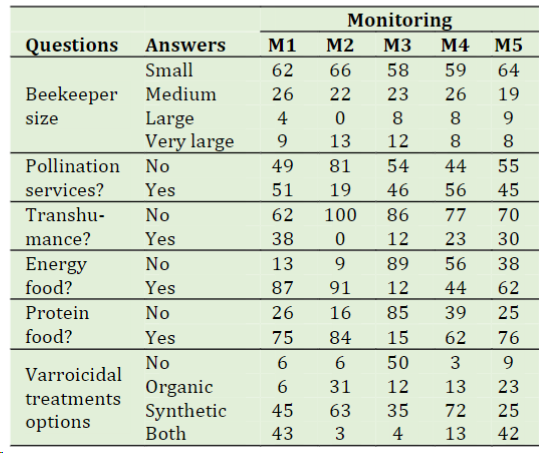
Table 1 shows the characterization of the surveyed beekeepers according to each monitoring. It is possible to observe, based on the data collected for the first monitoring (47 participants), that more than half of the beekeepers (62%) manage between 1 and 299 hives, considering themselves as small beekeepers. This number is followed by 26% of medium beekeepers (300-799 hives), and only a small percentage manage more than 800 hives (large and very large beekeepers). Nearly 50% of those beekeepers who responded to the question about providing pollination services said they did so, except for monitoring 2. Only a small percentage of beekeepers (between 12% and 39%) said they moved their colonies (transhumance).
Regarding food supplementation, 87% of the beekeepers supplemented their colonies with energy food, before wintering in 2015 (M1), while in the same period of 2016 (M4), only 44% % performed this action. This is explained by the availability of floral resources in ecosystems, dependent on climatic conditions. On the other hand, most of the beekeepers (between 62% and 84%) provided protein supplements during the autumn (M1 and M4) and spring (M2 and M5) seasons, while 15% did it in summer time (M3) (Table 1). The majority of beekeepers (between 89% and 97%) responded that they apply treatments against Varroa sp. during both the autumn and spring seasons, and a significant 50% also apply treatments throughout the harvest season (M3). It has been demonstrated that the application of varroicidal treatments results in the development of resistant mites and a reduction in their sensitivity to standard treatments (Gregorc et al., 2018; Higes et al., 2020), but also, it causes contamination of the bee products (Giorgini et al., 2023). This is applicable even for residues at sub-lethal concentrations (Qi et al., 2020).
The survey's findings indicate that beekeepers favor synthetic varroicidal treatments or mixtures of synthetic and organic treatments, with organic treatments alone appearing to be rarely employed by the beekeepers surveyed. In fact, the Chilean Health Service has approved the use of five medications for beekeeping. From them, only one is an organic product; the others are synthetic products using amitraz or flumethrin as active ingredients (SAG, 2020). It has been reported that, the use of these drugs induces stress in adult bees, disturbing the physiological balance in bees, causes acute toxicity to newly emerged honeybees and affects the development and survival of honeybees (Qi et al., 2020; Li et al., 2022).
Table 1 Characterizationof beekeepers according to the infor-mation of the survey for each monitoring (M1-M5). M1: April-May (autumn); M2: September-October (spring); M3: December-January (summer); M4: April-May (autumn); M5: September-October (spring). Results are expressed as the percentage of the total beekeepers in each monitoring
As a result of this circumstance and the lack of any other options, beekeepers are forced to repeatedly and continuously apply varroicidal treatments, stressing the colonies and shortening the lifespan of the bees (Zhu et al., 2015; Fisher et al., 2017). In this context, the use of artisanal formulations without doses or treatment plans developed with pharmacological rigor exacerbates this issue. On the other hand, it has been demonstrated that pharmacological interactions, such as those involving fungicides used in surrounding flowering crops, acaricides used to treat Varroa destructor, and antimicrobials to control bacteria and microsporidia in hives, can be extremely harmful (Johnson et al., 2013).
Infestation rates by Varroa sp. and their prevalence
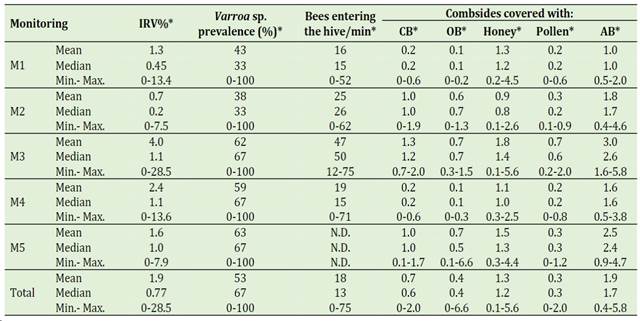
The infestation rates by Varroa sp. (IRV%) in adult bees were highly variable (between 0 and 29%), as presented in Table 2, and significant differences between monitoring were observed (Kruskal-Wallis test, p < 0.05). These rates exceed the tolerance thresholds previously reported (Verde et al., 2013; Giacobino et al., 2014 and 2016). As a result, some colonies would be at grave risk (particularly in monitoring 1, 3, and 5), although coexisting in the same productive environment as the populations with fewer parasites.
The prevalence (%) of Varroa sp. had little varia-tion during the monitoring, except in monitoring M2 compared to M5 (Kruskal-Wallis test, p < 0.05). It is worth mentioning that monitoring 5 (spring) obtained the highest prevalence of the study. This difference could be related to the reduction in the expression of the colonies’ immune mechanisms that occurs during wintering (Steinmann et al., 2015) and that would translate into higher infestation rates during spring. The global prevalence (%) for the study period was 53%, which is close to the prevalence reported previously for the country (SAG, 2020).
The infestation rates by Varroa sp. (IRV%) concerning the different categorical variables are presented in Figure 1. There are no significant differences in infestation rates regarding to the number of colonies managed by the beekeeper, nor when pollination services are provided or transhumance is carried out (Figure 1- A, 1-B, 1-C).
However, when the colonies are supplemented with energy or protein food the infestation rates are significantly lower than when no food supplements are provided (Figure 1-D and 1-E) (Mann-Whitney U test, p < 0.05). Similarly, the application of varroicidal treatments also contributes significantly reducing the infestation rates by this parasite, exposing values close to 4% when no treatments are applied (Kruskal-Wallis test, p < 0.05), as seen in Figure 1-F. It is important to note that Varroa sp. infestation rates vary depending on the type of treatment used. In example, the IRV% is lower when synthetic (1.7%) or organic (2.2%) treatments are used, or when both are used together, in which case the lowest rate of Varroa sp. infestation (1.0%) was seen.
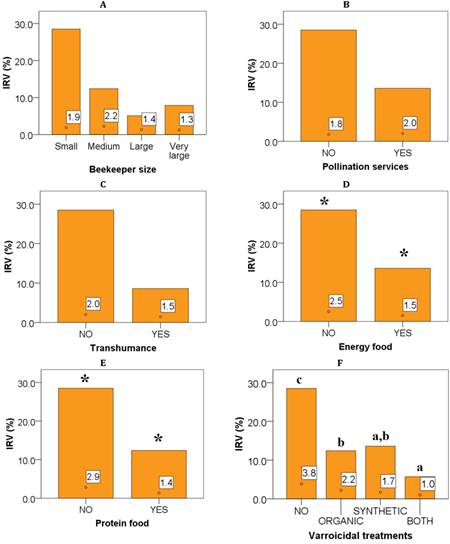
Figure 1 Minimum and maximum graphs for infestation rates by Varroa sp. (IRV%), and their mean values (white squares) according to: A) The size of the beekeeper, B) Pollination services, C) Transhumance, D) Energy supplements, E) Protein supplements, F) Application of varroicidal treatments. Significant differences are presented according to the Mann-Whitney U (*) or Kruskal-Wallis (a-c) test (p < 0.05).
These results coincide with the findings presented by Giacobino et al. (2018), who demonstrated that the life history of bee colonies is strongly affected by the management practices used by beekeepers, especially those related to the control of Varroa sp. (treatments) and supplementary feeding. Nutrition has been also shown to be a key factor for bee health (Steinhauer et al., 2018). The correct delivery of food supplements (carbohydrates and proteins) reduces the susceptibility of bees to biological agents since it increases the availability of food inside the colony (Sperandio et al., 2019). For their part, carbohydrate supplements can protect bees from pesticide poisoning (Tosi et al., 2017), and additionally, pollen supplementation stimulates colony growth and improves their survival, even in the presence of mites or viruses, due to their positive effect on bee immunity (DeGrandi-Hoffman et al., 2020). All these factors are components of good beekeeping management techniques that have been used throughout the world (Sperandio et al., 2019).
Honeybee colony strength
Table 2 shows that there were significant diffe-rences in the parameters related to honeybee colony strength among the five monitoring sessions (Kruskal-Wallis test, p < 0.01), with monitoring 3 being the time when the parameters were better because it was performed during the summer. When observing in detail, it is possible to notice that the number of comb sides with closed brood, open brood, pollen, and adult bees increases towards spring and then, from spring to summer, and then decreases during autumn. Another indicator of the colony strength was the number of bees entering the hive during one minute, which was highly variable (between 0 and 75 bees/min) according to the season. It is assumed that changes in the results are connected to both the time of year and the internal structure of the colonies because the number of bees entering the hive is closely correlated with the internal population of adult bees (Grant et al., 2021).
It is noteworthy that controlling parasites largely depends on the used treatments. This finding reflects the absence of integrated management strategies in the country, which prevent the re-infestation of the colonies. Thus, colonies from the same apiary or nearby colonies may interact due to their flying radius. It is still unclear how different factors can explain why bee colonies show a consistent correlation between the level of infestation rates with their clinical condition and the subsequent evolution of their colony strength (Bernardi et al., 2016; Wegener et al., 2017; DeGrandi-Hoffmann et al., 2017).
In this sense, it can be taken as a reference to the Cuban honeybee health model. It is focused on stopping the epidemiological chain of Varroa sp., which affects all bee colonies in that country. This model has a preventive approach. It started with a territorial organization of the managed bee populations (Verde et al. 2012; Verde et al., 2013). The program is aimed at preserving or restoring the internal balance of bee families and bee populations regarding to productive ecosystems, based on good management practices by the beekeepers. The productive performance and exportable quality of honey in that country is the expression of controlled parasitic rates, with an average of more than 50 kg of honey per colony each year in that country, and infestation rates by Varroa sp. less than 3%, in territories where treatments have not even been applied for more than two years (Sanabria et al., 2015).
An efficient strategy to control the infestation rates by Varroa mites and its consequences for bee populations should include a series of additional considerations to the current use of medications (SAG, 2020). It has been shown that the use of alternative organic treatments, applied according to the environmental conditions and nutritional status of the bee colonies, can be effective at a lower cost (Rashid et al., 2020).
Table 2 Characterizationof beekeepers according to the information of the survey for each monitoring (M1-M5). M1: April-May (autumn); M2: September-October (spring); M3: December-January (summer); M4: April-May (autumn); M5: September-October (spring)
Other strategies include a biological approach to control Varroa sp. mites populations (Gabel et al., 2023). In this sense, it can be mentioned the hygienic behavior of the honeybees, which is related to the removal of mite-infested brood (Danka et al., 2013; Panziera et al., 2017; Kirrane et al., 2018), but also, contributes to increasing honey production (Masaquiza et al., 2021). On the other hand, using a strain of bees that are resistant to mites, it is possible to use selective breeding to develop a dominant genetic component, as well as genetic advancements (Locke, 2016) or, take advantage of the characteristics of Africanized bees (Guzmán-Novoa et al., 2020; Jack and Ellis, 2021; Pinto et al., 2022; Castilhos et al., 2023). However, it is essential to include aspects that holistically address the control of parasitosis, considering the epidemiology of the mite and its territorial distribution. Therefore, to combat varroosis it is essential to have a series of biotechnical measures, good management practices, bee queens from certified origin, as well as, the application of geographically coordinated miticide treatments to improve the bee health and sustainability (Woodford et al., 2023). Only in this way, the treatment intervals can be lengthened and parasitic infestation rates reduced, safeguarding the safety of the obtained products as well, a key goal in the production of food for human consumption (Verde et al., 2013).
CONCLUSIONS
Several factors are associated with the epide-miological process of varroosis. According to the present results, the infestation rates by Varroa sp. are related to some management practices. Lower infestation rates were obtained when colonies were supplemented with energy and protein food, and when efficiently varroicidal treatments were applied. However, a high percentage of the surveyed beekeepers apply varroicidal treatments throughout the year, without a territorial program. Thus, it can be concluded that the populations under study display an epidemiological mosaic, determined mostly by uncoordinated control activities, which distorts any natural manifestation of the epidemiological behavior of the parasitosis. As a result, to effectively combat this parasitosis, additional territorial strategies must be included and managed by the respective health authorities, and beekeeper organizations. Holistic management should include selection and genetic improvement, parasite control biotechniques, and in some circumstances, coordinated territorial treatments.












 uBio
uBio 


