Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Neuro-Psiquiatría
versión impresa ISSN 0034-8597
Rev Neuropsiquiatr vol.78 no.3 Lima jul. 2015
Alzheimer’s disease: Toward the rational design ofan effective vaccine.
Enfermedad de Alzheimer: Hacia el diseño racional de una vacuna eficaz.
Dante J. Marciani 1,a
1 Qantu Therapeutics, Inc. Lewisville, Texas, USA.
SUMMARY
The promising clinical results with the human monoclonal antibodies aducanumab and solanezumab targeting β-amyloidin Alzheimer’s diseasetreatment, confirm both the amyloid cascade hypothesis and protective naturalimmunity, while strengthening the immunotherapeutic approach. That aducanumab recognizes a conformational epitope formed byoligomers emphasizes the need for whole β-amyloid, not just its B-cell epitopes as have been the norm to avoidpro-inflammatory Th1-reactions.That truncated β-amyloid having N-terminal pyroglutamate ispresent only in diseased brain simples a new useful vaccine antigen. Anotherrelevant antigenis the tau protein, which shows aclose association and cooperativity with β-amyloid in exacerbating this disease. Hence, effective vaccines may be polyvalent, presenting to the immune system a number ofantigens relevant to induce an immune response to prevent or slowdown the onsetof this disease. The presence of both B and T cell epitopes in the antigens,require a sole Th2 immunity to avert brain inflammation; a task that cannot beattain with adjuvants that under any conditions induce Th1 and/or Th17immunities. Hence, new vaccine adjuvants are need to safely induce Th2 whileinhibitingTh1 immunity, an objective that can be achieved with certain fucosylated glycans or triterpene glycosides, which apparently bind to the DC-SIGNlectin on dendritic cellspolarizingthe immune response toward Th2 immunity. Because the triterpeneglycosides have the pharmacophore needed toco-stimulate T cells, they may ameliorate the T-cell anergyassociated with immunosenescenceand responsible forpoor vaccine efficacy in the elderly population, a critical issue for anAlzheimer’s vaccine.
KEYWORDS: Alzheimer’s, immunotherapy, vaccines, adjuvants, immunomodulators
RESUMEN
Los resultados prometedores en el tratamiento de la enfermedad de Alzheimer con Aducanumab y Solanezumab, anticuerpos monoclonales humanos contra β-amiloide, ratificanla hipótesis de la cascada del amiloide y la existencia de inmunidad natural contra Alzheimer, mientras refuerza el método inmunoterapéutico. Que Aducanumab reconoce un epίtopo conformacional formado por oligómeros, acentúa la necesidad del β-amiloide completo y no solo sus epίtopos de células B, como ha sido la norma para evitar reacciones pro-inflamatoriasTh1. De que el β-amiloide truncado con piroglutamato en su extremo N-terminal se encuentra solo encerebros enfermos, es un antígeno útil; otro antígeno importante es la proteína tau, que tiene una estrecha asociación y cooperatividad con β-amiloideen exacerbar esta enfermedad. Una vacuna eficaz puede ser polivalente parapresentar al sistema inmunológico una variedad de antígenos importantes e inducir una respuesta para prevenir o retardar el comienzo de esta enfermedad. La necesidad de epítopos decélulas B y T en los antígenos, implica una inmunidad tipoTh2para evitar inflamación del cerebro; objetivo que no se puede alcanzar con adyuvantes que inducen inmunidades Th1 y/oTh17. Consecuentemente, se necesitan nuevos adyuvantes de vacunas para inducirsin riesgos la inmunidadTh2 mientras se inhibe la Th1, objetivo que se puede lograr con ciertos glicanos fucosiladoso glucósidos triterpénicos que se unen a la lectina DC-SIGN en células dendríticas, polarizando la respuesta hacia la inmunidad Th2. Como los glucósidos triterpénicos tienen el farmacóforo necesario para co-estimularlas células T, podrán moderar la anergia de las células T asociada con immunosenescencia, responsable por la baja eficacia de las vacunas en la población anciana, materia critica para vacunas contra la enfermedad de Alzheimer.
PALABRAS CLAVE: Alzheimer, inmunoterapia, vacunas, adyuvantes, inmunomoduladores.
INTRODUCTION
After decades of failures in the treatment of Alzheimer’sdisease (AD), the promising clinical results obtained during a phase Ib trial with the human monoclonal antibody (mAb) aducanumab, from Biogen (1), have brought optimism while showing thatthe immunotherapeutic approach is apparently the most promising one to prevent/treat this disease. Indeed, those initials results were supported by thesubsequent clinical studies with solanezumab (EliLilly), a humanized mAb thatbinds the central epitope of monomeric Aβ, preventing its aggregation and slowing the disease progress (2). Those results besides validating the immunotherapy’s method, also confirm the"beta-amyloid cascade hypothesis", which for years has provided thescientific bases to explain this disease and develop drugs to treat it (3); anotion that due to the numerous clinical failures, has been questioned. These results’ relevance is emphasized by the current number of worldwide ADcases, about 40 million, which is expected to be over 80 million by year 2040,and after double every 20years (4); obviously an outcome of the increased longevity as a result of modern medicine. Hence, this alarming raise of thisworldwide epidemic demands near term solutions, to prevent and/or delay theonset of AD. While various approaches to develop drugs for AD are beingpursued, those based on a protective immunological response are the most sound,i.e. studies have shown that humans at an early age start to produce antibodies against the protein beta-amyloid (Aβ), a response that decreases with age as the incidence of AD starts toincrease (5).The presence of these protective anti-Aβ antibodies has been confirmed by the studies with aducanumab, which is a replica of antibodies present inolder by mentally sound human beings (1).Yet, this immune response also occursin non-humane primates and other animals like dogs and cats, which show aneurodegenerative process similar to AD (6,7). While there are rare cases whereAD is due to mutations of the Aβ gene, this diseaseor sporadic AD is largely a result of the aging process.
The Aβ cascade hypothesis
Germane to AD is the Aβ cascade hypothesis (ACH), which over twenty years ago proposed that the deposition of aberrant Aβ aggregates as plaques on neuronal cells was the primary cause of their death. Since then and because brain damage was observed beforeplaque formation and other findings, the hypothesis has gone through severaliterations that include Aβ soluble forms, which are also toxic to brain cells (3,8). In fact, Aβ shows a complex cascade of conformational and oligomerizationstages that lead to the formation of neurotoxic forms, which may involve otherproteins (Figure 1). Aβ consistslargely of two isoforms, amain peptide called Aβ40 that has aminoacid residues from 1 to 40 and a minor one that is less than 5% of the Aβ, which has 2 extra amino acids and is named Aβ42. While both isoforms have a tendency to form β-sheets, because Aβ42 has more aggregability than Aβ40, it seems that it starts the process leading to theformation of oligomers, fibrils and plaques (9). A feature of Aβ is the unique spatial structures formed by its aberrant aggregates, i.e. conformational epitopes that are independent from its aminoacid sequence. Indeed that proteins classified as amyloids, Aβ being one of them, while they do not share amino acidsequence homologies have a similar generic conformational epitope, has beenshown using mAbs(10). Of interest is that these proteins are frequently associated with amyloidosis, a group of diseases characterized by protein misfolding or proteinopathies (11,12).
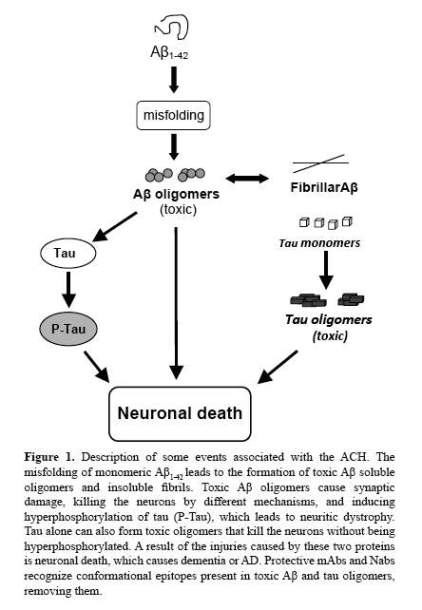
The products resulting from the changes taking place at the Aβ cascade are this protein’s aberrant conformations and oligomers, showing different degrees of stability and cytotoxicity. Since Aβ belongs to the amyloid proteins,i.e. small proteins lacking ordered structures and with tendency to formamyloid fibrils rather than crystals; its assembly is complex and diverse, which can be affected by the presence of other proteins and lipids, and is characterized by extensive polymorphism (12). Following extracellular releaseof the Aβ monomer and its interactionswith GM1 ganglioside or either ApoJor αB-crystallin proteins, itforms a variety of pre-fibrillar soluble Aβ oligomers that differ in sizeand are more cytotoxic than fibrillar Aβ (13). Hence, it´s now well established that soluble Aβ oligomers are more relevant for AD than fibrillar Aβ; indeed cytotoxic Aβ oligomers can kill thecells by a variety of mechanisms, finding that pinpointsto these forms as the therapeutic target rather than the fibrillar forms.This proposal is supported by the priondiseases, where misfolding of amyloidogenicpeptides lead to neurotoxicity without plaque formation(14).
Though the aducanumab and solanezumab results strongly support the crucial role of Aβ in AD pathology, proteins liketau may have also a role in this disease. In effect, tau is affected invitro by sub-nanomolar concentrations of solubleAβ dimers isolated from the brains of AD patients, whichinduce its hyperphosphorylation that disrupts themicrotubule cytoskeleton, causing neuriticdy strophy; an effect that can be blocked by anti-Aβ antibodies(8). In fact, earlier in vivo studies showed that intra-cerebral infusion of youngmice with Aβ containing brain-extracts fromaged mice induced taupathology in APP x Tautransgenic mice (15). As both proteins are physically inclose contact and have significant interactions; itis very possible that they may act in a concerted manner, magnifying theirpathological effects (Figure1). Proof for the interactions between Aβ and tau is provided by studies using a mouse model with a genetic knock-out for tau protein, which showed thatabsence of tau in knock-out mice prevents the synaptic dysfunction caused byacute exposure to Aβ 42 (16) and that tau actsdown stream of Aβ, as proposed by Hardy and Selkoe. Indeed, tau can exert its neurotoxicity alone or ina cooperative manner with Aβ. While it was assumed thatonly hyperphosphorylated tau was neurotoxic, newevidence shows that like Aβ, tau oligomers rather thanthe fibrils are the toxic forms regardless of their phosphorylation(17). Hence, due to the close interactionsamong Aβ and tau in AD pathology, anoption would be to have both proteins as therapeutic targets, as have beensuggested (18). While thefunctions of Aβ and its precursor protein,APP, are being elucidated, apparently their normal forms play a role in neural cell development andsurvival,plus enhancing memory; functions apparently dependent on the proteinfolding and concentrations. Thus, therapeuticstrategies should distinguish thegood from the badprotein species, to prevent complications due to therapy.
Immunity and Alzheimer’s disease
Several studies have shown the production by healthy people of autoantibodies (Nabs) against Aβ, production that starts at avery early age and that after being maintained through most of the life, begins to decrease with aging and is quite lower in AD. That the NAbs’ decrease occurs when AD starts to develop implies a neuroprotective role of immunity against AD. In effect, a retrospective study had shown that previous treatment with intravenous immunoglobulin (IVIG) reduces the risk of AD by 42% (reviewed in 19),significant when it is consider that the donorpopulation is made of young, healthy adultsthat supposedly have higher levelsof Aβ antibodies. Later studiesshowing that NAbs react with oligomericbut not monomeric Aβ, imply that these antibodies by targeting toxicoligomers rather than the non-toxic monomers protect against AD. One study alsoshowed that deliveryof NAbsto an AD mouse model reduced plaque formationand ledto improvement of the mice behavior, confirming these antibodies’ protectiverole against AD (20). As NAbs recognize oligomeric but not monomeric Aβ, it is evident that theyrecognize a conformational epitope that must be common to the differentoligomers. Because this epitopeis independent of Aβ’s amino acid sequence, but dependent on the newspatial structure shaped by the assembly of several monomers into oligomers, its presence would require thewhole proteinmolecule and not just some partialpeptides.
Convincing supportfor the protective anti-Aβ Nabs is provided by theresults of the passive immunotherapy study with aducanumab,a humanrecombinant IgG1 mAbcloned from aged donors that were cognitively normal and thus have effectivelyresisted AD. Like protectiveNabs, this mAb binds selectively and with high affinity to Aβ soluble oligomers andinsoluble fibrils, but not to monomers (1). Althoughthere is no information about the nature of the epitope recognized by aducanumab, as it only recognizes aggregated forms of Aβ, it is most likely aconformational epitope like that described for Nabs. From this clinical studyand previous animal studies, though not well understood it is clear thatthe natural autoimmune response differentiates between "goodmonomers" and "badoligomers" of Aβ; which suggests a biologicalrole for monomeric Aβ. While several mechanisms havebeen proposed to explain the antibody-mediated removal of Aβ, the one for aducanumab seems to involve microglial-mediatedphagocytosis and clearance of Aβ through the IgG1, whichlargely depends on the effector function of the mAb(1). This mechanism requires passage of the antibodies across the blood-brainbarrier (BBB) and into the CNS, an immune privileged site; passage that due tothe BBB would be limited, which would explain the high doses of this mAb needed to achieve a therapeutic effect, as compared tothe doses needed for mAbs acting outside the CNS.
Although the focusof passive immunotherapy has been the IgG immunoglobulins, IgMs also play arole in the removal of Aβ, but by a different mechanismthat does not involveeither microglia or macrophages.The IgMs exert their anti-Aβ activity by catalyticallydegrading this protein,i.e.these antibodies are highly specific proteases that recognize Aβ at concentrations lower thanthose required for IgG binding (21). As due to theirsize, ~ 900 KDa, it is unlikely that IgMs would pass across the BBB, they act by degrading theperipheral Aβ that has been transportedacross the barrier as a complex with anti-Aβ Ig Gs.That the content of catalytic IgMs,relative to the other antibodies, increases with age points to theiraging-induced synthesis that is present even in those affected by AD; probablyto maintain the capacity to process the increasing amounts of toxic Aβ forms associated with aging.Due to the irrelative lylong life in circulation(half-life approximately 5 days), coupled to the fact that one IgM can process several Aβ molecules, catalytic IgMscan be effective therapeutic agents for AD (22). Yet,catalytic IgMs are not limited to AD, but they arealso present in other autoimmune conditions, apparently a quick protectiveresponse against aberrant autoantigens. Since the IgM response is T-cell independent and devoid of memoryT-cells, it is unlikely that these catalytic properties would be passed to IgG antibodies after the IgM → IgGclass switching; indeed, natural anti-Aβ IgGpreparations and IgG mAbshave negligible proteolytic activity. Hence,catalytic IgMscan be considered an innate immunityrather than an adaptive immunity mechanism.
While an anti-Aβ antibody response or humoral immunity isusuallyassociated with positive effects on the prevention or slowing down ofAD, a pro-inflammatory response or T-cell immunity isusually linked to damaging effects on the CNS. Although humoralimmunityis linked to one type of T helper cells, i.e. Th2, the pro-inflammatoryimmunity is linked to two different types of T helper cells,i.e. Th1 and Th17. In Th2-biased immunity, besidesstimulation of antibodyproduction, there isproduction of anti-inflammatory cytokines, e.g. IL-4 andIL-10; while Th1-biasedimmunity is characterized by the pro-inflammatory cytokines, e.g. IL-2 and IFN-γ, and theproductionof several effector cells, like CD8+ Tcells orcyto toxic T lymphocytes (CTL), activated macrophages andothers. A new type of T helper cell is Th17, which induces an inflammatoryimmunity mediated by pro-inflammatory cytokines IL-17 and IL-22;while Th17immunity provides a strong anti-microbial response at the mucosalbarrier,it is often associated with damaging inflammatory responses and tissueinjury in several autoimmune conditions, e.g.multiple sclerosis and rheumatoidarthritis (23).Thatsome agents that elicit Th2 immunity can induce Th17 in the absence of Th1immunity, is an important issue in the design of AD vaccines.
While there isconsensus that in AD an inflammatoryimmunity ismostly damaging, animal studies have delivered different mechanismstoexplain such immune response; apparently the result of factors like the animalspecies and/or their different strains used in the studies, aspreviouslyreported. Still, a variable that can significantly impact thoseresultsis the adjuvant or immune modulator used to stimulate an immuneresponse;i.e. most studies assume that all Th1 adjuvantselicitingan inflammatory response have similar mechanisms of action. But, theevidence from infectious diseases and cancer vaccinesshows that such is notthe case. Actually, althoughalmost all of the known adjuvantsinduce Th1 immunity,a result of the induction of adaptive immunity followingthestimulation of innate immunity, their mechanism of action is dependent onthe innate immunity receptor involved, like the toll likereceptors (TLRs)(24). Proof of these differentmechanisms is provided by the fact thatconcurrentstimulation of different TLRs, results in a synergistic effect onthe immune response due to interactions between differentand independentimmune modulatory pathways. Thissituation mayexplain the different results fromanimal studies, and the failures in developinganeffective AD vaccine for humans.
Though a discussionof inflammation and AD is beyondthis review, someissues relevant to active immunotherapy will be brieflyaddressedhere. While accepted that inflammation has a damaging role in AD, a sitinvolves highly interacting molecular mediators and mechanisms, some of thembeneficial, plus the lack of a clear insight about thisresponse, it may bedifficult and even risky tobroadly interfere with it. Still, findings like Aβ’s intrinsiccapacity to induce astrocytes and microglia to secrete in vitro and invivo pro-inflammatory cytokines, like IL-1α, IL-1β, IL-6 and TNF-α, are important to developimmunotherapeutic methods (25). Thispro-inflammatoryactivity may be explained by A B being an endogenous "danger-associatedmolecular pattern", which binds to innate immunityreceptor(s)on astrocytes and microglia, to initiatean innateimmune response that triggers an adaptive inflammatory response. Indeed, Aβ induces in vitro theexpression by microgliaof TLR-2 and TLR-4, TLR-2being the microglia’s primary receptor for Aβ; binding of Aβ to TLR-2, would initiate a neuroinflammatoryactivation, which could explain some of Aβ damaging effects(26). Anotherinflammatory cytokine that apparently aggravates AD is IFN-γ, i.e. Aβ-specific CD4+ Th1 cellsadoptively transferred to an AD mouse model increased microglia activation andAβ deposition, effects that were attenuated with an IFN-γ antibody (27). Yet, this studyshowed that while Th2cells had beneficial effects, the Th17 cells did not causedamage, despite being strongly inflammatory.
Evidently, differentfrom infectious diseases andcancer, where the desiredresponse is usually pro-inflammatory Th1, the response needed in AD is sole humoral Th2 immunity.Thus, itwould be useful to induce Th2 while inhibiting, but not abrogating, theinflammatory Th1 immuno response; difficult as mostimmune modulators induce both Th1 and Th2 immunities. Thus, development ofan effective and safe vaccine to prevent and/or treat ADwould require an approach closely mimicking the natural protective immunity.
Alzheimer’s disease – Passiveimmunotherapy
The clinicalresults with aducanumaband solanezumabsupport both passive and activeimmunotherapeuticapproaches to prevent/treat AD. In passive immunotherapy, anantibodypreparation like IVIG or a mAb against a nantigen, e.g. Aβ or tau, is administered to apatient to achieve thedesired therapeutic effects.The benefit of passive AD immunotherapy is itsquickeffect, regardless of the recipient’s immune competence; useful with theelderly that usually has an immune decline due to aging. Theadministrationschedule and dose needed would depend on the mAb’snature;i.e. IgGs have a circulating half-life of approximately20 days, but, due to the BBB only a small fraction enters theCNS.Yet, that ultrasound may transiently open the BBB and allow passage ofantibodies from the blood into the CNS, could allow amore effective delivery of the mAbs to the brain(28). Of interest is thecontrast between the clinicalresults from aducanumaband those from the mAbs, bapineuzumaband gantenerumab (29). Bapineuzumabisa humanized mAb that binds the N-terminal 5-aminoacidresidues of fibrillar and soluble Aβ, presumably disruptingaggregation but, it failed inclinical trials. Incontrast, the human mAb gantenerumabbinds to a conformational epitopemade by the N-terminaland central amino acids of aggregated Aβ; acting presumably by takingapart and recruitingmicroglia to degrade Aβ plaques. Yet, gantenerumabalthough similar in most of its properties to aducanumab,did not show clinicalbenefits.
It is difficult toexplain the differences between theaducanumab and gantenerumabclinical results, because while there issignificant information about the epitope recognized by gantenerumab(29),all that is known about aducanumab is that itis a conformational epitope found in Aβ soluble oligomers and insolublefibrils, but not in monomers(1). As aducanumabwasisolated directly from humans, it possible recognizes a generic amyloid fibrilepitope such asthose found in other pathogenicamyloids. A characteristic of these conformational epitopes isthatthey are sequence independent and dependent only on the aberrantamyloids’ aggregates; i.e. many misfoldedamyloidogenicproteins share these conformational epitopes (30). Thus, it is possible thatthe body hasprotective antibodies against theseproteins, which eliminate them before theycausedamage; a protective mechanism that decreases with age as result of immunosenescence. Indeed, amyloidosisofwhich AD is one of them, has been called a biological aging problem as wellas a disease. Hence, the different outcomes of thesestudies may be due to the methods used to identify those antibodies; i.e. aducanumabwas the result of an approach that took advantageof the natural selectionprocess when they used aged,healthy and mentally competent individuals as donors (1). But,the efficacy of these mAbs maydependon various factors, i.e. solanezumab while deliveredpoor results in the first clinical trial (29), it gavepositive results in a subsequent study (2). Yet,the presence of protective antibodies against aberrant forms of otherwisenormal proteins, besides confirming the validity of passive immunotherapy, alsoprovides strong supportto vaccination as a preventionand/or treatment against AD.
Alzheimer’s disease – Active immunotherapyor vaccination
Natural protectiveimmunity indicates a possible useof passive andactive immunotherapy to prevent and/or treat that disease, a notion with a longhistory of support from the infectious diseases and cancerareas.Hence, aducanumab and solanezumab’spositive results have provided a proof that was missing in AD. While vaccinationwas the first immunotherapeutic approach tried in AD, a series offailures raised doubts about its feasibility as well asthe science behind it.Thus, proposals to rationallydevelop an effective AD vaccine would need tomake aretrospective analysis of past studies to identify the reasons for their failuresand justify new approaches. That due to large differences betweenvaccinesfor AD and infectious agents little can be transfer to AD vaccines,can explain these disappointments, despite thepresence of a natural protectiveimmunity.
Sub-unit ADvaccines have an antigen(s) and anadjuvant or immunomodulator. While the antigen isrequiredto induce a specific immune response, the adjuvant is the componentthatwill stimulate and bias such a response; i.e. the adjuvant not only jumpsstart the immune system, but it also decides in whichdirection will go. As the adjuvant properties are independent from theantigen’s nature, alterationof an antigen toelicit a safer immune response would not help. A concern forADvaccines is that adjuvants generally stimulate a pro-inflammatoryTh1, which is always present with a humoralTh2 immune response; i.e. adjuvantsthat induce asole Th2 immunity are rare (31).Adjuvants exerttheir immune modulatory activities byusing differentbut highly specific receptors andpaths to stimulate immunity; but as mostimmunologicalreceptors are linked to innate immunity, e.g. TLRs and NOD-likereceptors(NLRs), usually the initial innate immunity response will be follow apro-inflammatory adaptive immunoresponse.
The Aβ42 AN1792 vaccine, Thefirst ADvaccine containing Aβ42 and clinically tested, AN1792, showed in a phase 1study a large variability in antibody response, butno side effects. While ithas been reported that thephase 1 response was predominantly Th2, it isdifficultto accept that conclusion since QS-21 is a Th1 adjuvant (32); hence, those results may indicate its de-acylation, whichwouldresult in Th2-biased immune response. But, sinceduringthe phase 2 study, after1 to 3 immunizations some patients developedencephalitis, the study was ended.Yet, the phase 2antibody responders, i.e. 20%, regardless of the encephalitis, had antibodies against the linear N-terminal Aβ 1-8 peptide; a response independentof conformation or aggregation.While the encephalitishave been rightly attributed to the adjuvant QS-21, the different outcomes ofphase 1 and 2 area result of the enhancing effects of thenon-ionicdetergent polysorbate 80 on QS-21’s adjuvanticity (31); a difference that may have been made even more evident by a possible de-acylation of QS-21inthe phase 1 study.
Interpretation ofthe results from cell mediate dimmunity induced by theAN1792 vaccine is intricate, due to the differences in the immune responsebetween humans and mouse models. The postmortem studies ofAN1792 vaccinatedpatients have shown presence of CD4+T cells, CTLs and macrophage infiltration, as well as clearance of amyloidplaques,due to vaccination; yet, it is clear that Aβ-specific CTLs did not have arole in the encephalitisprocess (33). In contrast,CD4+ T cell shave an important role secretingcytokinesthat induce inflammation; one of such cytokines with a unique positionis IFN-γ, which at high levels in thebrain is damaging, but at low levels helps neuronal repair. Indeed, adoptivelytransferredTh1-producing CD4+ T cells increase microglia activation and Aβ deposition that are associated with impaired cognitive function, while thoseproducing Th2 or Th17 did not cause anychanges. Aquestion is why AD vaccines are effective in mouse models, but notin humans? A reason that has been offered is that vaccinesin transgenic mice are working in a preventive mode while in humans are in atherapeutic modeafter onset of AD; i.e. it seems tobe a matter of timing.
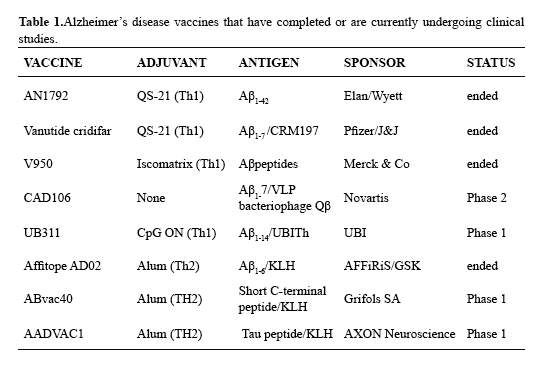
Truncated Aβ vaccines, an outcome of the damagingresultsobtained with the AN1792 vaccine has been to modify the Aβ42 antigen by deleting all ofthe T-cell epitopes, leaving only the N-terminal B-cell epitope, but mostlyusing Th1 adjuvants;yet, theGrifols’ vaccine has a short C-terminal peptideconjugated to KLH with alum Table 1). However, Th1 adjuvants regardless of the antigencan elicit a systemic inflammatory response that may act at the BBB to inducedamaging immunological reactions affecting the CNS (34). Thus, it is verylikely that CD4+ T-cells activated by Th1 adjuvants would secrete inflammatory cytokinesthat affect the CNS, regardless of Aβ lacking the T-cell epitopes. Another problem with truncated Aβ antigens is their induced antibody response, which targetsmonomers and plaques that are not effective therapeutic targets. Indeed, thatmany older people that have amyloid plaques are intellectually competent showsthat plaque is not necessarily the cause of AD; i.e. it has been proposed thatplaque is a way for the body to remove toxic Aβ. Yet, truncated Aβ peptides were used before the sequence-independentconformational epitopes in Aβ were identifiedusing mAbs, since amyloids cannot be crystallized foranalysis by X-ray crystallography (10,12).Nonetheless, if until recently the roles for monomeric and oligomericAβ in neurotoxicity were uncertain, the aducanumab study has showed that monomeric Aβ is not the right therapeutic target to prevent or treatAD.
Different from mostN-truncated Aβ peptides, those having pyroglutamateas an amino terminal group at positions 3 or11, Aβ N3 (pE) and AβN11(pE)respectively, are potentially effective targets for immunotherapy (35). Thesemodified peptides are more toxic than plain Aβ and their presence correlateswith AD presence, i.e. they are absent from normal brains. Their value has beenshown by passive immunotherapy, i.e. the treatment of transgenic mouse modelsfor AD with mAbs directed against the terminal pyroglutamate reduces the Aβ plaque load and lower thelevels of AβN3(pE)and AβN11(pE) forms (36). Hence, itis possible to consider these forms as likely vaccine antigen, perhaps in apolyvalent vaccine where a number of potentially relevantAβ antigens are present.
Because of the pastimmunotherapy problems and the promising results with aducanumaband solanezumab, is evident that an effective ADvaccine may require whole Aβ42 to form the conformationalepitope that is found in soluble Aβ oligomers and fibrils, but notmonomers, as well as some truncated Aβ forms like AβNx(pE)(35). Studies with the canine model for AD (6), which is more similar to humanAD than the transgenic mouse models, have shown that long-term vaccinationsresult in a progressive antibody response that drifts with time fromrecognizing linear Aβ epitopes to conformationalones (37). Thus, it is doubtful that an early anamnestic response willbe helpful in AD vaccines, as the initial antibodyresponse has no benefit in AD prevention and/or treatment; which explains whysurvivors vaccinated withAN1792, despite having for years high antibody titersand clearance of plaque, did not show any amelioration of the disease(33).Hence, development of an ADvaccine will requirean unconventional approach.
Toward the rational design of an AD vaccine
Several lessonsfrom the previous AD vaccine and aducanumab studiescan be applied to this vaccine’s development; particularly in view thatmethods from infectious disease vaccinesmay not bedirectly applicable to AD vaccines. Also, that this vaccine’s requirements,like a need for whole Aβ and apparently tau with T and B-cell epitopesin a vaccine where a prerequisite issole Th2 immunity, are rather conflicting, show the need for new strategies.While the Nabs and aducanumab studies have shown are quirement for the whole Aβin order to present that conformational epitope crucial to induce a protective immunity, th estudies with the AD caninemodel has revealed the complex chain of eventsleadingto that response. Here we would address the roles of the vaccine’santigen and adjuvant, as well as strategies to elicit a sole Th2 immunity andprevent neuroinflammation.
Aβ42 – the antigent that protective NAbs and aducanumab cannot recognize Aβ monomers indicates that thereis no involvement of linear epitopes, usually identified by epitopemappingusing short overlapping peptides of the antigen; which may be due to the amyloidogenic nature of this protein (12). Amyloids form fibrils, where the initial event is protein misfolding; theseaberrant forms can start extensive oligomers and fibrils, which are associatedwith the amyloids’ toxicity. Indeed, monomericAβ in aqueous solutions formaggregates with an intermolecular hydrogen bonded structural motif shared bypathogenic amyloids, which as indicated before is sequence independent (38); anepitope(s)that presumably is recognized by aducanumaband Nabs. Although preparations of AB42 show a wide variety of oligomers,the canine vaccinestudies have shown that these preparations can be suitablevaccineantigens. Yet, it is possible that the production of stable oligomers with wellconserved structures may be attained byintermolecularcross-linking and other alternative methods discussed later.Thus,the issue is how to use an antigen with the T and B-cell epitopes needed toform the conformational epitope that would stimulate production of protective antibodies,in a vaccine where Th1 immunity cannot be tolerated? The pragmatic approach toinduce safely such immune response would be by using Th2 adjuvants; a challengeas most adjuvants induces Th1 with Th2 and/or Th17 immunities.
A viablealternative to the Aβ42 antigen would be the use ofpeptide analogs that mimic the generic epitope(s) found in oligomericand fibrillar Aβ (39); analogs that should bemore stable andreproducible than Aβ preparations. A disadvantageof these antigens couldbe the induction of a narrow erimmune response against generic epitopes than thatinduced by whole Aβ; a possibility that needs tobe tested using different mAbs that recognize thenatural conformational epitope(s). Clearly, the main advantageof these peptide analogs will be reproducibility, as it is importanttoinduce early the correct immune response. Yet, the canine studies have shown thatafter prolonged immunizations, the immune system develops that protective immuneresponse, even when using heterogeneous Aβ preparations (40). Anotherissue frequently overlooked, is the role of anti-Aβ IgMsthat catalyticallydestroy this protein outside theCNS; but, different from Nabs and aducanumab, the IgMs recognize epitopes formed by specific Aβ amino acid sequences (21,22). Thus, IgM production willrequire vaccination with whole Aβ. Perhaps, an option would be apolyvalent Aβ vaccine having the antigensneeded to stimulate bothtypes of antibody response.Yet, regardless of the antigens, the immunityshouldbe Th2, which would require new adjuvants.
Th2adjuvants or immune modulators, it is evident that the AN1792 vaccine damaging effectswere caused by the adjuvant QS-21; a glycoside isolated from Quillaja saponariaMolina, a tree native to Peru and Chile. QS-21 is one of the few Th1adjuvants that work without involvement of innate immunity receptors and itacts on T-cells and antigen presenting cells, i.e. macrophages and dendriticcells (DC), inducing the production ofCD4+ Th1 and CTLs (32). As discussed, thereportedly safe Th2 response induced by AN1792 during phase 1, points toproblems with the original vaccine, i.e.de-acylation of QS-21, a chemicalchange that shifts the response from Th1 to Th2 immunity (41); a situationdifficult to determine without cytokine analysis.
Adjuvants are usually recognized by the immune system as a signal that the body is underattack by pathogens, which triggeraTh1 immuno response.A fitting response against pathogens, but not for self-antigens such as Aβ, as an inflammatory responsewill cause organ damage. As adjuvants induce a systemic and not just a local immuneresponse to fight infections trough the body, it isunlikely that deleting anantigen’s T-cellepitopes will avert that response. An effect of a systemic Th1 immunity is that the inflammatory cytokines might activate the BBB endothelial cells to secreteinflammatory mediators into the brain, initiating or aggravating inflammation,which is an undesirable situation in AD (42). Yet, while Th1 adjuvants are not acceptable in AD vaccines, because of the immune decline associatedwithaging, some kind of adjuvant would be needed to induce and maintain aneffective Th2 immune response in the elderly.
Th2anti-inflammatory adjuvants are rare and their origins seem to coincide withthat of humoralimmunity well over 200 million yearsago. In fact, Th2 adjuvants are related to products made by parasitic helminths, which by inhibiting the host’sinflammatory response and boosting themilder humoral one, assure their survival and long-termco existence with the host (43). Interestingly, these compoundsinhibit but do not eliminate the pro-inflammatory Th1 immunity, needed for protection against pathogens and incipient tumors. Helminths producetwo types of Th2 immune modulators, one made of proteins and lipids that have phosphorylcholine (PC) and another that include various fucosylated glycans. Presentlythere is an experimental AD vaccine with PC as a Th2 adjuvant, but as PCderivatives are water insoluble this vaccine requires liposomes(42),which may limit its accessibility in parts of the world. Also, PC may induceunder some conditions Th17 immunity that is linked to inflammation in autoimmune diseases; in contrast, fucosylated glycans are water soluble and easy to formulate. These glycans work by binding to DC-SIGN, a DC’s C-type lectin, biasing DCs toward Th2 immunity while inhibiting the inflammatory Th1 immuno response (45). Thus, fucosylated glycans with Aβ42 should elicit aTh2 antibody response and systemicanti-inflammatory immunity. Yet, their synthesis is costly.
By serendipity, thede-acylated derivative of QS-21 named QT-0101is aneffective Th2 adjuvant that different from QS-21is stable and significantlyless toxic. Its mechanism of action maybe explainedby the fact that de-acylation of QS-21frees its single fucosylresidue, which presumably becomes available to bind to DC-SIGN and bias DCstoward Th2 immunity. Like thehelminths’ fucosylated glycans, it does notabrogate Th1 immunity, butinhibits it in a reversiblemanner (46). That QT-0101facilitates the passage of proteins across mucosaewould allow nasal deliverywhile avoidingintra muscular injections, as injections are hard on the elderly that have lessmuscle mass than younger people. This situation becomes acute with the longterm immunizations, needed because of the immune decline associated with aging.
Since the neurological changes, decline in memory andimmunologicalresponse in aging dogs, parallel those seeing in AD, the long-term vaccinationstudies with the canine model using Aβ42 are relevant to thedevelopment of a human vaccine.The canine vaccineused alum, a safe but weak Th2 adjuvant (40), as shown by the influenza vaccinethat is highly effective in the young, i.e. around 80 to90 percent protection,but it has a low efficacy in the elderly, i.e. as low as30 percent. Moreover, alumdoes not induce a systemic anti-inflammatory response that would be of benefitin AD. Another problem associated with aging is immunosenescence, where T cells and DCs lossreceptors and ligands thatare needed for T cell activation and prevent anergy.Thus, an AD vaccinewould benefit from an adjuvantlike QT-0101, which in addition to elicitinga strongsystemic Th2 immunity, may prevent T-cellanergy bydelivering via its aldehydegroup the co-stimulatorysignal needed for T-cell activation. Nonetheless, the prolonged vaccination of dogs with Aβ42 plus alum resulted in anantibody response that shifted fromrecognizing themonomeric Aβ N-terminal region to one thatrecognized a sequenceindependent conformation alepitope (37). In fact, the late antibody response is similar to that found in Nabs and probably in aducanumab. Yet, there was no cognitive improvement in thevaccinatedas compared to non-vaccinated dogs.
While many reasonsmay exist to account for thedifferent results between the vaccinated dogs and aducanumabstudies, aconspicuous one is the amount of antibodies found in vaccinated dogsversus that used in the mAbstudy. Vaccinated dogshad IgGlevels of around 37 µg/mL of plasma (37), while a single aducanumab treatment delivers to the patient enough mAb to reacha level of 257µg/mL of plasma, or 10 mg/kg(47), i.e. 7 times higher. Another factorwould be the antibody’saffinity for its epitope,i.e.aducanumab was chosendue to its high affinity; while there is noinformation about the avidity of the dog antibodies, it is unlikelythatit would be high, as alum does not induce an effective antibody affinitymaturation process. Therefore, that the different results may be due to theanti body concentrations and their avidity raises the possible that a vaccine inducing high levels of high avidity antibodies will show beneficial effects(48).Thus, a vaccine should have the right adjuvant to induce Th2anti-inflammatory response with production of antibodies with a high avidity for the antigen. This situation suggests that vaccines may be more effective used in a preventive mode in the younger immunecompetent population, rather than as therapeutic agents in the aged population suffering of immunosene scence. It is quite possible in view of the new development sand lessons from the past, that there would be an end to the streamof disappointments in AD drug-development (49,50).
CONCLUSIONS
The promising clinical studies with the mAb aducanumabshowing that thisdrug reduces amyloid plaques andimproves cognitive functions, strengthen the immunotherapeutic approach, whileconfirming the key role of Aβ in AD pathologyandexistence of a natural protective immunity against Aβ toxic forms. These findingsalso support the use ofvaccines to prevent and/ortreat AD, an approach that has yielded onlyfailures.Evidently, an AD vaccine would need Aβ42 as an antigen to allowformation of the conformation-dependent epitope, like that recognized by aducanumab.Indeed, these results were confirmed in thecanine model, which showed thatupon vaccination, theantibody response shifted from linear epitopestofinal conformational epitopes; but, there was no improvement of the cognitive functions, a result that may be due to the loweranti body concentration compared with that of aducanumab.Thus,a vaccine should have besides Aβ42 and potentially tau protein, a strong sole Th2 immunity adjuvant to induce an elevated production ofantibodies with high affinity for the antigen, a response that alum may notdeliver. Novel adjuvants based on PC and fucosylatedcompounds could fill that need; especially QT-0101, a fucosyl glycoside with anti-inflammatory properties, which may also ameliorate T-cell anergy that is quite common in the aging population andresponsible for a poor immune response.
Hence an effective AD vaccine should have Aβ42, which apparently isprocessed by the immune systemin various ways,synthetic analogs of that generic epitope(51), plus the N-truncated Aβ forms with pyroglutamicacid as the terminal residue, which are foundonly with AD and clearly have a role in the disease pathology. Due to the close relation between Aβ and tau in this disease progression, tau may be considered as another antigen. Thus, the vaccine should be able to elicitimmune responses against different but relevant antigens, i.e. the vaccinewould be apolyvalent vaccine with one caveat, because of its various self-antigens having all of their T-cell epitopes,theonly safe and apparently required immune response would be a sole Th2 and preferentially if the Th1 immunity is inhibited, but notabrogated. This way the vaccine will induce a Th2 immune response against the antigens and aconcomitant systemic anti-inflammatoryimmunity, which will be beneficial in amelioratingtheinflammation associated with aging. It is expected that the vaccine would perform better in the younger population, i.e. below65 years of age, than in the older one with more than 70 years of age; hence, as most vaccines it wouldbe more effective when used in a preventive rather than therapeutic mode. Considering what it is known about the immunopharmacology of adjuvants, it is evidently that use compounds that under any circumstances induce Th1 or Th17 immunities, would result in damagingside effects.
REFERENCES
1.Keller DM. Finally, a big win for amonoclonal in Alzheimers. AD/PD 2015: International Conference on Alzheimers and Parkinsons Diseases; 2015. Available in: http://www.medscape. com/viewarticle/841856
2.Brooks M. Solanezumab shows potential disease-modifying effect in AD. Alzheimers Association International Conference 2015; 2015. Available in: http://www.medscape.com/viewarticle/848445
3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimers disease: Progress and problems on the road to therapeutics. Science. 2002; 297:353-356. [ Links ]
4.Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimers and vascular types. Biomed Res Int. 2014;2014: 908915. http://dx.doi.org/10.1155/2014/908915
5.Britschgi M, Olin CE, Johns HT, Johns HT, Takeda-Uchimura Y, LeMieux MC, et al. Neuroprotective natural antibodies to assemblies of amyloid ogenicpeptides decrease with normal aging and advancing Alzheimers disease. Proc Natl Acad Sci USA. 2009; 106:12145-12150. [ Links ]
6.Davis PR, Head E. Prevention approaches in a preclinical canine model of Alzheimers disease: benefits and challenges. Front Pharmacol.2014;5:47. [ Links ]
7.Philipson O, Lord A, Gumucio A, OCallaghan P, Lannfelt L, Nilsson LNG. Animal models of amyloid-β-related pathologies in Alzheimers disease. FEBS J. 2010; 277: 1389-1409. [ Links ]
8.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012; 2(7):a006338. doi:10.1101/cshperspect.a006338. [ Links ]
9.Younkin SG. Evidence that A beta 42 is the real culprit in Alzheimers disease. Ann Neurol. 1995;37: 287-288. [ Links ]
10.ONuallain B, Wetzel R. Conformational Abs recognizing a generic amyloidfibril epitope. Proc Natl Acad Sci USA. 2002; 99: 1485-1490. [ Links ]
11.Merlini G, Belloti V. Molecular mechanisms of amyloidosis. N Engl JMed. 2003; 349: 583-596. [ Links ]
12.Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006; 27: 570-575. [ Links ]
13.Sakono M, Zako T. Amyloid oligomers: formation and toxicity of Aβ oligomers. FEBS J. 2010;277: 1348-1358. [ Links ]
14.Gandy S. The role of amyloid beta accumulation in common forms of Alzheimers disease. J Clin Invest. 2005; 115: 1121-1129. [ Links ]
15.Bolmont T, Clavaguera F, Meyer-Luehmann M, Herzig MC, Radde R, Staufenbiel M, et al. Induction of tau pathology by intracerebral infusion of amyloid- β-containing brain extract andby amyloid-β- deposition in APP x Tautransgenic mice. Am J Pathol.2007; 171: 2012-2020. [ Links ]
16.Shipton OA, Leitz JR, Dworzak J, Acton CE, Tunbridge EM, Denk F, et al. Tauprotein is required for amyloid β-induced impairment of hippocampal long-term potentiation. J Neurosci.2011; 31: 1688-1692. [ Links ]
17.Marciani DJ. Alzheimers disease vaccine development: A new strategy focusing on immune modulation. J Neuroimmunol. 2015; 287: 54-63. [ Links ]
18.Wisniewski T, Boutajangout A. Vaccination as a therapeutic approach for Alzheimers disease. Mt Sinai J Med. 2010; 77:17-31. [ Links ]
19.Marciani DJ. Development of Alzheimers disease vaccines: a perspective. Austin Alzheimers J Parkinsons Dis. 2014; 1(1): 4. [ Links ]
20.Dodel R, Balakrishnan K, Keyvani K, Deuster O, Neff F, Andrei-Selmer LC, et al. Naturally occurring autoantibodies against β-amyloid: investigating theirrole intransgenic animal and in vitro models of Alzheimers disease. J Neurosci. 2011; 31: 5847-5854. [ Links ]
21.Taguchi H, Planque S, Nishiyama Y, Symersky J, Boivin S, Szabo P, et al. Autoantibody-catalyzed hydrolysis ofamyloid β peptide. J Biol Chem. 2008; 283:4714-4722. [ Links ]
22.Planque SA, Nishiyama Y, Sonoda S, Lin Y, Taguchi H, Hara M, et al. Specific amyloid β clearance by a catalytic antibody construct. J Biol Chem. 2015; 290: 10229-10241. [ Links ]
23.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells inorgan-specific autoimmunity. J Autoimmun. 2008; 31:252-256. [ Links ]
24.Akira S. Innate immunity and adjuvants. Phil Trans R Soc B. 2011; 366:2748-2755. [ Links ]
25.Szczepanik AM, Rampe D, Ringheim GE. Amyloid-β peptide fragments p3 and p4induce pro-inflammatory cytokine and chemokine production in vitro and in vivo. J Neurochem. 2001;77:304-317. [ Links ]
26.Cappellano G, Carecchio M, Fleetwood T, Magistrelli L, Cantello R, Dianzani U, et al. Immunity and inflammation in neurodegenerative diseases. Am J Neurodegener Dis. 2013; 2: 89-107. [ Links ]
27.Browne TC, McQuillan K, McManus RM, OReilly JA, Mills KHG, Lynch MA. IFN-γ production by amyloid β-specific Th1 cells promotesmicroglial activation and increases plaque burden in a mouse model of Alzheimers disease. J Immunol. 2013; 190: 2241-2251. [ Links ]
28.Leinenga G, Gotz J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimers disease mouse model. Sci Transl Med. 2015;7(278):278-33. [ Links ]
29.Prins ND, Scheltens P. Treating Alzheimers disease with monoclonal antibodies: current status and outlook for the future. Alzheimers Res Ther. 2013; 5(6): 56. [ Links ]
30.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble oligomer simplies common mechanisms of Pathogenesis. Science. 2003; 300: 486-489. [ Links ]
31.Marciani DJ. New Th2 adjuvants for preventive and active immunotherapy of neurodegenerative proteinopathies. Drug Discov Today. 2014;19:912-920. [ Links ]
32.Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov Today. 2003; 8:934-945. [ Links ]
33.Zotova E, Bharambe V, Cheaveau M, Morgan W, Holmes C, Harris S, et al. Inflammatory components in human Alzheimersdisease and after active amyloid β42 immunization. Brain. 2013; 136: 2677-2696. [ Links ]
34.Erickson MA, Dohi K, Banks WA. Neuroinflammation: A common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012; 19:121-130. [ Links ]
35.Perez-Garmendia R, Gevorkian G. Pyroglutamate-modified amyloid beta peptides: Emerging targets for Alzheimers disease immunotherapy. Curr Neuropharmacol. 2013;11:491-8. [ Links ]
36.Frost JL, Liu B, Kleinschmidt M, Schilling S, Demuth HU, Lemere CA. Passive immunization against pyroglutamate-3 amyloid-b reduces plaque burden in Alzheimers-like transgenic mice: a pilot study. Neurodegenerative Dis. 2012; 10: 265-270. [ Links ]
37.Vasilevko V, PopV, Kim HJ, Saing T, Glabe CC, Milton S, et al. Linear and conformation specific antibodies in agedbeagles after prolonged vaccination with aggregated Abeta. Neurobiol Dis. 2010; 39: 301-310. [ Links ]
38.Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008; 283: 29639-29643. [ Links ]
39.Roeder AM, Roettger Y, Stündel A, Dodel R, Geyer A. Synthetic dimericAβ(28-40) mimics the complex epitope of a human anti-Aβ autoantibodies against toxic Aβ oligomers. J Biol Chem. 2013; 288: 27638-27645. [ Links ]
40.Head E, Pop V, Vasilevko V, Hill MA, Saing T, Sarzosa F, et al. A two-year study with fibrillar β-amyloid (Aβ) immunization in aged canines: Effects on cognitive function and brain Aβ. J Neurosci. 2008; 28: 3555-3566. [ Links ]
41.Marciani DJ, Pathak AK, Reynolds RG, Seitz L, May R. Altered immunomodulating and toxicological properties of degraded Quillaja saponaria Molina saponins. Int Immunopharmacol. 2001; 1:813-818. [ Links ]
42.Pan W, Stone KP, Hsuchou H, Manda VK, ZhangY, Kastin AJ. Cytokine signaling modulates blood-brain barrier function. Curr Pharm Des. 2011; 17: 3729-3740. [ Links ]
43.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites-masters of regulation. Immunol Rev. 2004; 201: 89-116. [ Links ]
44.Carrera I, Etcheverría I, Fernández-Novoa L, Ruggero V, Lombardi M, Lakshmana MK, et al. A comparative evaluation of a novel vaccine in APP/PS1 mouse models of Alzheimers disease. Biomed Res Int. 2015; 2015: 807146. doi: 10.1155/2015/807146. [ Links ]
45.Thomas PG, Harn DA. Immune biasing by helminth glycans. Cell Microbiol. 2004; 6: 13-22. [ Links ]
46.Wang Y, DaDara AA, Thomas PG, Harn DA. Dendritic cells activated by an anti-inflammatory agent induce CD4+T helper type 2 responses without impairing CD8+ memory and effect orcytotoxic T-lymphocyte responses. Immunology. 2009; 129: 406-417. [ Links ]
47.Jeffrey S. More positive data on aducanumab in Alzheimers. Medscape; 2015. http://www.medscape.com/viewarticle/844502. [ Links ]
48.Eisen HN. Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol Res. 2014; 2: 381-392. [ Links ]
49.Cummings JL, Morstorf T, Zhong K. Alzheimers disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014; 6(4):37. [ Links ]
50.Lannfelt L, Relkin NR, Siemers ER. Amyloid-b-directed immunotherapy of Alzheimers disease. J Intern Med. 2014;275: 284-295. [ Links ]
51.Rasool S, Albay R, Martinez-Coria H, Breydo L, Wu J, Milton S, et al. Vaccination with a non-human random sequence amyloid oligomer mimic results in improved cognitive function and reduced plaque deposition and micro hemorrhage in Tg2576 mice. Mol Neurodegener. 2012 ;7:37. doi:10.1186/1750-1326-7-37. [ Links ]
Acknowledgments
I would like toexpress my gratitude to Dr. Alberto Cazorla Talleri, whom 30 yearsago waskind enough to search and procure in Peru an authentic sample of thecortex from the soap bark tree, which allowed togetherwith my associates thedevelopment of the adjuvantQS-21.
Corresponding author
Dante J. Marciani
Recibido:06/07/2015













