INTRODUCTION
The dystrophinopathies are a set of X-linked muscle diseases ranging from mild to severe. These include Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and DMD- associated dilated cardiomyopathy (DCM) (1, 2). DMD is the most common dystrophinopathy and affects approximately 1 in every 6000 live births with a prevalence of 3.52-12.57 per 100,000 individuals 3,4. DMD is characterized by rapidly progressive muscle weakness, average age of 4 years at the time of diagnosis, and incapacitating weakness requiring the use of a wheelchair by 12 years. Life expectancy is ~20 years 5,6, DMD female carriers usually are asymptomatic, although they may also experience muscle weakness, left-ventricle dilation and, rarely, cramps and dilated cardiomyopathy 7,8.
By the 1970s-80s, carrier detection and corresponding genetic counseling in DMD was achieved through Bayesian statistical methods using the pedigree and the creatine kinase values of female relatives. Depending on their level of risk, women could decide on either abstaining from having children or the sterilization of a spouse to reduce the DMD incidence in relatives with a positive family history 9. Currently, the DMD-carrier status is determined through a genetic test, MLPA or sequencing if needed 10,11.
In Peru, based on an MLPA analysis, a DMD research study lead by the Centro de Genética y Biología Molecular (CGBM) of the School of Medicine of the Universidad San Martin de Porres, has supported the molecular diagnosis of more than 100 DMD and BMD cases since 2012 12. However, to date, no genetic counseling program has been implemented for oligosymptomatic or asymptomatic carriers.
The aim of this report is to describe the first genetic counseling experience in Peru for the diagnosis of the DMD-carrier state of an at-risk woman. Thus, we analyze our intervention retrospectively; highlighting our successes and misunderstandings, in order to improve the counseling and follow-up of the asymptomatic carrier of DMD.
Case report
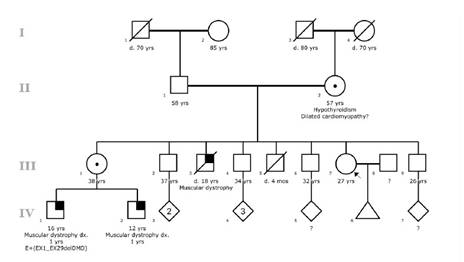
A 27-years-old woman (Individual III-7 of Figure 1), came to the Clinic Center in search of genetic counseling. She has a clear DMD family history: a brother (Individual III-3 of Figure 1) who died at 16 years of age with a DMD phenotype; and two nephews also affected (Individual IV-1 and IV-2 of Figure 1), one of them (Individual IV-1 of Figure 1) with molecular diagnosis of DMD showing a large deletion through exons 1-29 that included the promoter region DP427c of the DMD gene.
The consultand and her sister (Individual III-1 of Figure 1) were actively providing care for her SMS- affected nephews. She has witnessed the significant efforts aimed at preventing complications in the affected family members. It is due to this that she expressed great concern about the possibilities of her having children affected with DMD.
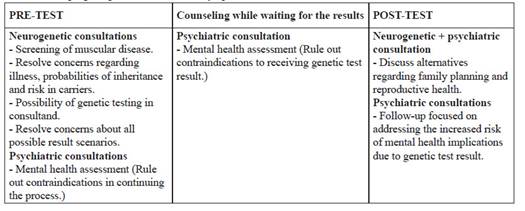
The consultand underwent a genetic counseling pilot program for diagnosis of asymptomatic carriers of DMD, program which consisted of three periods: Pre-test counseling (before the molecular test), counseling while waiting for results, and post-test counseling (after delivery of the result).
Pre-test Counseling
The consultand attended her first genetic counseling sessions alone, receiving then a detailed information about the disease; the inheritance pattern, the progression of the disease, and the risk to family members. The general assessment resulted in a normal physical and neurological examination and a normal electromyography. Cardiological evaluation was also normal, creatine kinase (60 U/L) and lactate dehydrogenase (262 U/L) levels were within normal ranges. We then concluded that there was no evidence of muscular disease. The staff resolved all the questions and concerns made by the consultand. She was then offered asymptomatic carrier testing. Counselling at this point included information regarding the test, purpose, limitations and timeline for the results. She was informed that our team would remain available for ongoing counseling throughout the entire process. Thereafter, the consultand attended meetings accompanied by her partner and both were eager to know the risk of DMD in future pregnancies.
Recurrence risk was reviewed in detail, including the options they would have if the consultand was a carrier.
In coordination with a university-affiliated molecular testing laboratory, the staff explained the possibility of participating in an asymptomatic-carrier diagnosis pilot program. The consultand agreed to participate in the genetic counseling sessions and other complementary evaluations including psychiatric support. She underwent psychiatric evaluation in order to identify any mental condition or disorder, such as anxiety, major depression or risk of suicide, in need of treatment or stabilization before continuing with the pilot program. There was no anxiety, depressive symptoms or suicide risk, and psychotherapy was recommended throughout the process.
The entire pre-test counseling took about 14 months, due to long waiting times for routine lab tests and specialized clinical appointments, and unforeseen logistical problems at the molecular testing lab site. Meanwhile, we continued offering additional sessions to answer new questions and concerns from the consultand and her partner regarding possible outcomes of the genetic testing before taking the blood sample.
Counseling while waiting for the results
An additional psychiatric appointment was scheduled two months after the sample was taken. During this session there were expressions of mild anxiety and grief mostly related to the delayed delivery of lab results. The consultand demonstrated willingness to be open to all the possible outcomes and agreed to continue with the evaluations after receiving the results.
Post-test counseling
Fourteen weeks after taking the sample, the consultand was found to be a carrier of the familiar pathogenic variant in the DMD gene. The patient was scheduled within four weeks after receiving the results. A genetic counseling appointment took place to disclose the genetic test results. The session was led by a neurogeneticist,, and a psychiatrist was also present for additional support. The consultand and her partner were informed about the carrier status with a 50% risk of transmitting a DMD pathogenic variant to future offspring. The staff also provided counseling regarding family planning procedures like pre- implantation diagnosis, i.r., male or female embryos without the pathogenic variant that can be selected and transferred to the uterus 13, a procedure that, however, is currently very limited in Peru.
The couple was given time to decide on these alternatives and, 10 days later, during the psychiatric evaluation, the consultand reported that she was going through a grieving process due to the genetic test results. Despite being offered an appointment in three weeks, she did not attend until five months later. She later stated having problems with her partner regarding whether they would have children due to the risks implied. No more appointments regarding her asymptomatic status have been scheduled since then, even though she continues to accompany her nephews to their regular neurogenetic appointments.
DISCUSSION
We describe the first experience, within the Peruvian public healthcare system, of genetic counseling in an asymptomatic female carrier who was interested in knowing the risk of having children affected with DMD, the disease of two of her nephews. We proposed a pilot program (Table 1). This genetic counseling intervention allowed us to reflect on many aspects including carrier advice guidelines to local realities, optimal timeline for the process, and importance of post-test follow-up.
The Peruvian health system has limited access to genetic diagnosis and few healthcare professionals with training in medical genetics. Developing countries like Peru, provide only few public services for genetic diagnosis, mainly for some monogenic disorders and genetic risk for cancer. There are no accredited genetic counseling programs in the country; thus, this service is provided by medical geneticists and other professionals with training in genetics, mostly based in the capital city of Lima 14,15. According to international genetic counseling guidelines, these processes must be carried out by accredited and certified genetic counselors 16, thus, limiting the development of predictive diagnostic programs.
The prolonged time of the pre-test period generated understandable anxiety in the consultand. The specialty consultations (neurology and cardiology) and supplementary tests requested to rule out symptomatic status in the proband, lasted about 8 months due to the extended waiting periods for appointments in the public health system. Subsequently, the genetic counseling sessions and psychiatry consultations lasted about 2 months. By the end of this period, the test could not be performed due to a four-month delay of reagents importation for the lab, therefore the sample draw was performed about 14 months after the first pre-test counseling session. Despite this prolonged time period, the mild anxiety experienced by the consultand did not require pharmacologic treatment. It is not clear, however, if the prolonged waiting period generated a negative disposition against this program. Most presymptomatic tests are associated with anxiety or other behavioral disturbances, given their impact on decision making processes for the future 11,17. Thus, while implementing this program, we must make sure that other related hospital services are working regularly with a fully established timeline for responses.
The prolonged waiting time for the results increased anxiety and the post-test follow-up was discontinued. The delivery of results took about 14 weeks, and additional psychiatric consultation was scheduled for dealing with mild anxiety.
After the delivery of the results, the consultand only had one post-test counseling session where couple therapy was suggested but, unfortunately, she did not return for therapy afterward. Having the genetic testing available at the same institution offering the genetic counseling would have saved time and made this process more efficient.
From the entire process we can identify positive and negative aspects that must be respectively reaffirmed or improved in the future. The most important positive point is offering the consultands the possibility of making informed decisions in accordance to their own values, with regards to risk of disease recurrence in the family. International genetic counseling guidelines 18-20 agree that pre-symptomatic and carrier status diagnosis reduce mortality and disabilities secondary to genetic disorders. However, the extended period prior to taking the sample and the long waiting time for genetic testing generated anxiety. Another aspect to be improved is the post-test follow-up planification as we did not achieve appropriate long-term adherence. The implementing additional pre-test counseling sessions and neurology and cardiology follow-up consultation due to the increased risk for mild myopathy and cardiomyopathy 7,8, might improve this aspect of the program.
This experience allows us to reflect on the importance of implementing a standardized genetic counseling program for asymptomatic carriers of DMD. This first experience will serve as a basis for counseling programs in regions with limited resources. This experience will give us insights regarding challenges facing genetic counseling for adult onset inherited neurodegenerative disorders.