INTRODUCCION
COVID-19 is known to be caused by SARS-CoV-2, an RNA virus with a bilipid membrane containing proteins of crucial relevance for its pathogenesis(1, 2). SARS-CoV-2 is neuroinvasive, neurotropic, and neurovirulent. More than 460,000,000 cases, and around six million deaths due to COVID-19 worldwide3,4) and more than 3,500,000 cases and approximately 200,000 deaths(3,4. nationally, have been reported. SARS-CoV-2 incubation period is approximately 2 to 7 days, and the infection presents with symptoms such as fever, cough, fatigue, dyspnea, myalgia, and diarrhea5,6,7.
Guillain-Barré syndrome (GBS) is an autoimmune disease triggered by bacterial or viral infections8,9. It is postulated to be a disorder mediated by an antibody attack on the nerve axolemma, driven by molecular mimicry of microbial surface molecules10. Anti-LOS antibodies can bind to the structurally identical glycans present in ganglioside nerves, and anti-ganglioside subclass IgG1 and IgG3 antibodies are complement fixers that bind primarily to gangliosides GM1 and GD1a in acute motor axonal neuropathy8,10.
The primary manifestation of GBS is a progressive bilateral weakness that ascends from the lower extremities and can last up to 4 weeks. As well, GBS can involve areflexia, and potential cranial nerve involvement, resulting in symptoms such as numbness, tingling, and weakness, which may progress to paralysis. Other common symptoms are areflexia and cranial nerve involvement. Additionally, sensory symptoms, ataxia, and autonomic dysfunction can be present10.
The most common precipitant of GBS is gastroenteritis caused by Campylobacter jejuni: two case-control studies describe up to 26-27% of previous gastroenteritis due to C. jejuni compared to controls11,12. A report by Hao et al. places influenza A in second place with a 17% incidence. On the other hand, 12.4% of patients with GBS were positive for IgM cytomegalovirus12.
Similarly, symptomatic C. jejuni infection is associated with up to a 100-fold increased risk of developing GBS during a 2-month follow-up in a Swedish cohort; meanwhile, a case-control study resulted in an odds of up to 60 times in patients with C. jejuni enteritis13,14. In addition, the background of C. jejuni infection is associated with a worse prognosis, defined as a longer time to recover walking without help (90 days vs. 45 days) (11.
The Zika virus (ZIKV) outbreak has also been associated with GBS. IgM for ZIKV was positive in 93% of cases with GBS15. The calculated prevalence for this association was 1.23%16.
In Peru, a GBS outbreak occurred in mid-2019; some authors determined that the pure motor subtype (80%) was the most frequent. Most GBS cases were positive for C. jejuni IgM as well as for anti-ganglioside antibodies17. In a systematic review of case reports in 2020, it was suggested that the onset of GBS took place after the symptoms of COVID-19. In addition, the variants were associated with bilateral facial palsies, whose mechanism is a post-inflammatory syndrome18.
The mechanism behind the association between COVID-19 and GBS is not yet fully understood. However, preliminary studies suggest that the SARS-CoV-2 virus's neuroinvasive and neurotropic properties may play a role in triggering GBS. One theory suggests that SARS-CoV-2 infection can do it through molecular mimicry, where the virus's spike protein (S1) binds to the ACE2 receptor, and S2 fuses the viral membrane with the cell membrane2. This interaction may lead to an autoimmune response, causing the body to produce antibodies that can bind to the nerve axolemma and trigger GBS(19, 20).
Despite these findings, the current evidence falls short of establishing a definitive causal relationship between COVID-19 and GBS. The intricate interplay between viral infections and autoimmune responses requires more in-depth exploration. Further research is imperative to unravel the underlying mechanisms and identify specific risk factors contributing to the development of GBS in the context of COVID-19 infections. By elucidating these aspects, we aim to provide a more comprehensive understanding of the relationship between COVID-19 and GBS, contributing valuable insights to the ongoing discourse in the medical community.
Methodology
We searched for studies reported from December 2019 to April 2022.
Criteria selection:
Inclusion criteria: Observational studies such as case series and case reports, and experimental studies and clinical trials published in Spanish and English, Portuguese. We included the referred studies because at the time of search, these study designs were the most probable to find reliable information regarding SGB presentation in COVID-19 patients.
Exclusion criteria: Case-control, and cohort studies, reviews, commentaries, and editorials. Studies written in different languages as referred above. Studies that were no possible to retrieve.
Study selection:
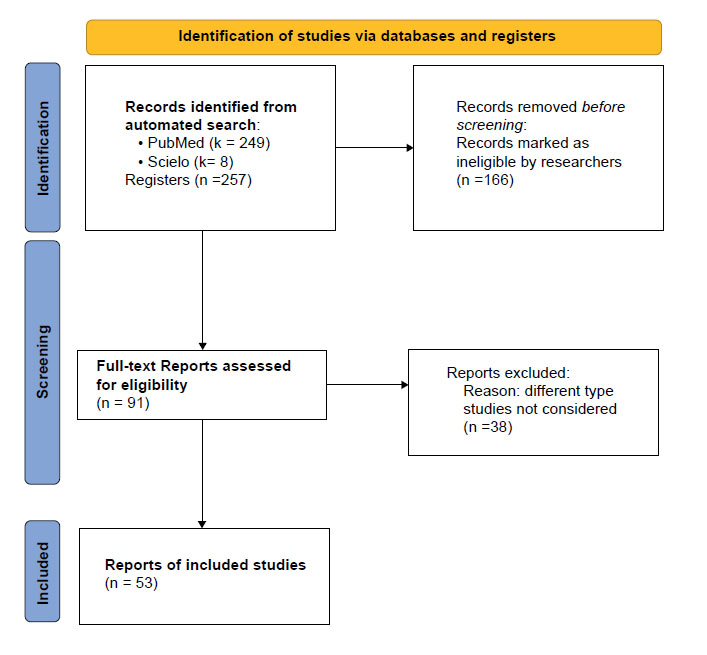
We used the NCBI and Scielo platforms for this automated search, which yielded 257 records. Of these, we removed 166 considered ineligible studies, i.e., those that not included keywords in titles or abstracts or different topics. We screened 91 articles for full-text consideration. We excluded 38 of them because they were of different types, not considered as inclusion criteria (Appendix 1). In addition, to search for a possible pathophysiological mechanism, the following MESH words were used: ( "Guillain-Barre Syndrome/etiology"[Mesh] OR "Guillain-Barre Syndrome/immunology"[Mesh] OR "Guillain-Barre Syndrome/pathology"[Mesh] OR "Guillain-Barre Syndrome/physiopathology"[Mesh] ) AND ( ("SARS-CoV-2/immunology"[Mesh] OR "SARS-CoV-2/pathogenicity"[Mesh] ) OR (SARS Coronavirus 2 OR Coronavirus 2, SARS OR Coronavirus Disease 2019 Virus OR 2019 Novel Coronavirus OR Coronavirus, 2019 Novel OR Novel Coronavirus, 2019 OR SARS-CoV-2 Virus OR SARS CoV 2 Virus OR SARS-CoV-2 Viruses OR Virus, SARS-CoV-2 OR 2019-nCoV OR COVID-19 Virus OR COVID 19 Virus OR COVID-19 Viruses OR Virus, COVID-19 OR COVID19 Virus OR COVID19 Viruses OR Virus, COVID19 OR Viruses, COVID19 OR Severe Acute Respiratory Syndrome Coronavirus 2)).
For the search of clinical manifestations, laboratory findings, and imaging studies of GBS in patients with SARS-CoV-2, the following keywords were used: (SARS Coronavirus 2 OR Coronavirus 2, SARS OR Coronavirus Disease 2019 Virus OR 2019 Novel Coronavirus OR Coronavirus, 2019 Novel OR Novel Coronavirus, 2019 OR SARS-CoV-2 Virus OR SARS CoV 2 Virus OR SARS-CoV-2 Viruses OR Virus, SARS-CoV-2 OR 2019-nCoV OR COVID-19 Virus OR COVID 19 Virus OR COVID-19 Viruses OR Virus, COVID-19 OR COVID19 Virus OR COVID19 Viruses OR Virus, COVID19 OR Viruses, COVID19 OR Severe Acute Respiratory Syndrome Coronavirus 2) AND ( "Guillain-Barre Syndrome/blood"[Mesh] OR "Guillain-Barre Syndrome/cerebrospinal fluid"[Mesh] OR "Guillain-Barre Syndrome/diagnosis"[Mesh] OR "Guillain-Barre Syndrome/pathology"[Mesh] ). We considered the first word building because it provided more records.
Results
In our search, 91 studies were identified, 38 of which were excluded because they did not accomplish the review objectives. Finally, we included 53 articles (Appendix 2) representing 72 cases (patients) since some studies reported more than one case.
The cases were classified according to COVID-19 diagnosis methods, symptoms, motor and sensory signs, autonomic alteration, antibodies profile, cerebrospinal fluid findings, imaging studies, types of GBS, and findings in nerve conduction studies.
COVID-19 diagnosis
Of the 72 cases, 77.68% (n=56) were diagnosed with SARS-CoV-2 infection through reverse transcriptase-polymerase chain reaction (PCR), and 1.39% (n=1) was diagnosed with rapid IgG antibody test. The diagnosis method was not mentioned in 20.83% (n=15) of the patients. However, these patients had high suspicion of COVID-19 due to respiratory symptoms.
COVID-19 symptoms
Of the 72 GBS cases with a history of COVID-19, the following symptoms were obtained: 45.83% (n=33) presented cough, 56.94% (n=41), fever, 22.22% (n=16), dyspnea, 16.67 % (n=12), dysgeusia with/without hyposmia, 9.72% (n=7), diarrhea, and 9.72% (n=7), headache.
Motor signs
Of the 72 patients, 34.72% (n=25) exhibited muscle weakness, with 27.78% (n=20) specifically experiencing weakness in the lower limbs, and 2.78% (n=2) in the upper limbs. Meanwhile, 34.72% (n=25) did not report global muscle weakness; such was identified in 11.11% (n=8) of the patients, the first neurological symptom being muscle weakness in the lower limbs. However, in the case reports, it is noteworthy that not all cases provide explicit details regarding the initial symptom. Additionally, only 6.94% (n=5) presented generalized hypotonia, and 2.78% (n=2) exhibited hypotonia exclusively in the lower limbs. Among the cases, 87.5% (n=63) reported no gait abnormality. Nevertheless, 6.94% (n=5) of patients displayed abasia, and 5.56% (n=4) presented paraparesis. Hypotonia was not reported in 90.28% (n=65) of the cases.
None of the patients who presented with muscle weakness in the lower or upper limbs have exhibited another neurological symptom at onset; however, 2 out of 3 patients who did not present muscle weakness debuted with hypotonia in the lower limbs, while one developed ataxia and areflexia. On the other hand, 3 patients who presented with generalized muscle weakness developed ascending paralysis at the onset.
In the deep tendon reflexes evaluation, 40.28% (n=29) presented global areflexia; specifically, 15.28% (n=28) presented areflexia in the lower limbs and 1.39% (n=1) in the upper limbs. The 19.44% (n=14) developed hyporeflexia in the lower limbs, upper limbs, and globally in 8.33% (n=6), 4.17% (n=3), and 6.94% (n=5) respectively compared to the 80.56% (n=58) of patients hyporeflexia was not reported. The only case that presented hyperreflexia did so in the upper limbs, representing 1.39% (n=1). Only 2.78% (n=2) of the patients showed bulbar weakness. Another patient presented a Babinski sign, 1.39% (n=1), and 5.56% (n=4) of the cases presented ataxia.
Sensory symptoms
16.67% (n=12) of the patients presented hypoesthesia in both lower and upper limbs. While 8.33% (n=6) presented it only in the lower limbs, and 1.39% (n=1) presented it only in the upper limbs. 73.61% (n=53) of the patients did not report this symptom. Only one patient presented hyperesthesia in the back.
Likewise, paresthesia was reported in both upper and lower limbs in 6.94% (n=5) of the patients, 20.83% (n=15) only in the lower limbs, and 2.78% (n=2) only in the upper limbs. The remaining 69.44% (n=50) did not report paresthesia. Back pain was also reported in 16.66% (n=12) of the patients.
Cerebrospinal Fluid (CSF) Findings
The mean CSF protein level was 140.23 mg/dL (SD: 106.71; Min: 37; Max: 620 mg/dL). Furthermore, albumin-cytologic dissociation was reported in 66.67% (n=48) of all cases observed. The absence of that dissociation was reported in 12.50% (n=9). However, the analysis for such dissociation was not reported in 20.83% (n=15).
Autonomic dysfunction
It was reported that 2.78% (n=2) of the patients presented fecal incontinence, while urination disturbances were reported in 6.94% (n=5) of all patients. Of the 72 cases, 2.78% (n=2) showed altered blood pressure same as 2.78% (n=2) of patients with impaired heart rate. Finally, only 1.39% (n=1) presented diarrhea.
Antibodies
Of the 72 reported cases, 31 were tested for antiganglioside antibodies, and 6.45% (n=2) of the patients had positive antibody titers. One of them had positive anti-GM2 and anti-GD3 IgM, and anti-GT1b IgG, while the other patient had positive anti-Gal-C antibodies.
Imaging findings
Plain radiography
Ten cases were reported with abnormal findings on chest X-rays: Infiltrates with alveolar pattern 10% (n=1), interstitial pattern 10% (n=1), alveolar-interstitial 10% (n=1) or unspecified pattern 70% (n=7), characteristically diffuse 20% (n=2), distributed bilaterally 60% (n=6) or unilaterally 20% (n=2). In addition, multilevel degenerative changes were found on a spinal radiograph.
Computed tomography
Seven cases were reported with abnormal findings on chest CT: Ground-glass opacities with bilateral distribution 57.14% (n=4) and located at basal 28.57% (n=2) or subpleural 14.29%(n=1) areas. On a brain CT, chronic ischemic encephalomalacia was found in the right occipital lobe.
Magnetic resonance imaging
Eleven cases with abnormal MRI findings were reported. In brain T1-weighted images, there were 9 cases of enhancement of cranial nerves such as optic 22.22% (n=2), oculomotor 22.22% (n=2), trigeminal 11.11% (n=1), trochlear 11.11% (n=1) and vagus 11.11% (n=1) nerves, as well as enhancement of associated structures like the olfactory bulb 11.11% (n=1) and Tenon's capsule 11.11% (n=1). In brain T2-weighted images, hyperintensity of the left oculomotor nerve 50% (n=1) and inflammation of the right hippocampus 50% (n=1) were found.
Spinal T1-weighted images showed enhancement in five structures: cervical leptomeninges at 28.57% (n=2), brainstem at 28.57% (n=2), C6-C7 nerve roots at 14.29% (n=1) or posterior nerve roots from T11 14.29% (n=1), and cauda equina 14.29% (n=1). In spinal T2-weighted images, alterations were found in 3 structures: hyperintensity of T7-T12 thoracic medulla 25% (n=1), stenosis of cervical 25% (n=1), and lumbar 50% (n=2) canals.
Types of Guillain-Barré Syndrome in the presence of COVID-19
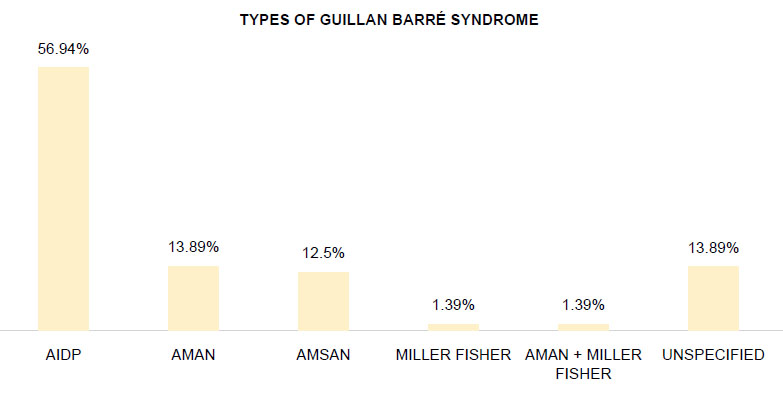
The most frequent type reported was Acute inflammatory demyelinating polyneuropathy (AIDP) presented in 56.94 % (n= 41) of all cases observed (Figure 1). The second most frequent type was Acute motor axonal neuropathy (AMAN), which represented 13.89% (n=11). The third type was Acute motor and sensory axonal neuropathy (AMSAN) in 12.50% (n= 9). Likewise, a Miller Fisher type 1.39% (n=1) and an overlap between AMAN and Miller Fisher 1.39% (n=1) were reported. Finally, 13.89% (n=10) of cases did not specify the type of GBS.
Findings of nerve conduction study
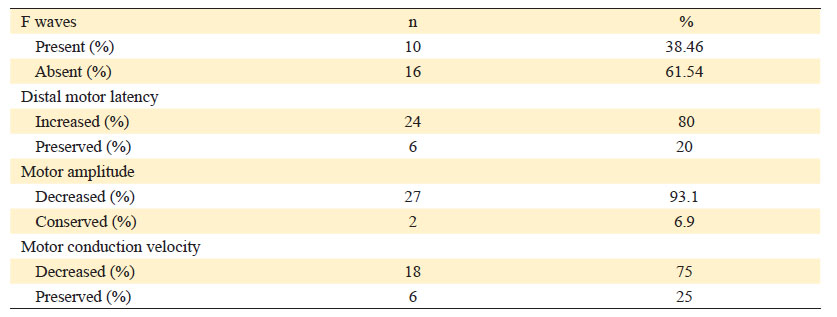
These findings were subclassified into the presence of F waves, distal latency, motor conduction amplitude and velocity, and nerve involvement (Table 1).
F waves
Twenty-six analyses of F waves were reported, of which 38.46% (n=10) presented F waves, and 61.54% (n=16) were absent.
Distal motor latency
Thirty results of distal motor latency were reported, 20% (n=6) maintained it preserved, while the remaining 80% (n=24) presented increased latency.
Motor amplitude
Twenty-nine analyses of motor amplitude were reported, 93.1% (n=27) of them presenting decreased motor amplitude, while 6.9% (n=2) remained preserved.
Motor conduction velocity
Twenty-four analyses of motor conduction velocity were reported, 75% (n=18) of them presented decreased velocity, and 25% (n=6) remained within the reference values.
Type of nerve
Nerve involvement was reported in 38 studies. They were classified into three groups: upper limbs, lower limbs, and cranial nerves (Table 2). In the upper limbs, median (n=19), ulnar (n=17), and radial (n=1) nerves were involved. In the lower limbs, the nerves involved were tibial (n=28), peroneal (n=23), and sural (n=1). The cranial nerves (CP) involved were CP III (n=1), CP VI (n=1), CP VII (n=2), and CP VIII (n=1). It should be noted that more than one type of nerve was involved in the same patient. In 34 studies, no nerve involvement was reported.
Table 2 Affected nerves according to the characteristics of nerve conduction studies*
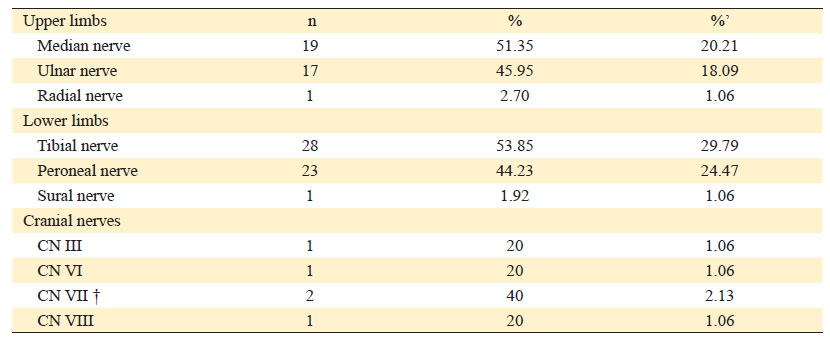
* There were patients with more than one nerve involved.
† Involvement of CN VII (facial nerve) was found clinically in 28 patients, described in more detail in Figure 2.
% Percentage based on the classification of affected nerves.
%’ Percentage based on the involvement of all affected nerves.
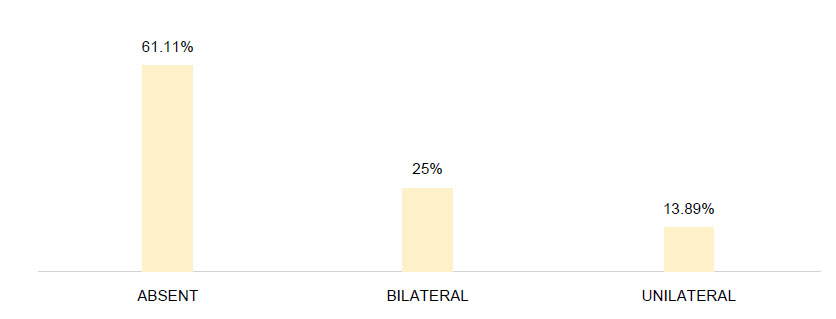
Facial palsy was presented by 38.89% (n=28) of the patients, of which 25% (n=18) was bilateral, and 13.89% (n=10) was unilateral (Figure 2).
In addition, it was reported that 11.11% (n=8) of the patients presented dysphagia, while the other 88.89% (n=64) did not.
GBS treatment
Of the 72 cases, treatment was only reported in 65 patients, of which 92.31% (n=60) received intravenous immunoglobulin, while 4.62% (n=3) and 3.08% (n=2) received plasmapheresis, and intravenous immunoglobulin with plasmapheresis, respectively.
Discussion
Pathophysiology of GBS associated with COVID-19 and its relationship with the results
Within the hypotheses of coinfection with SARS-CoV-2, autoimmunity is proposed as a mechanism of molecular mimicry, and a parainfectious phenomenon dependent on the cytokine storm caused by the COVID-19 condition. (21,22 The first hypothesis is supported by the development of symptoms between 3 days to 6 weeks after the respiratory infection, cytological albumin dissociation, PCR negativity for SARS-CoV-2 in the CSF, and improvement by treatment with intravenous immunoglobulin. (21,23 On the other hand, the cytokine storm hypothesis maintains that, following the intracellular invasion of SARS-CoV-2, active replication begins, generating new viral copies that induce pyroptosis of the infected cell and the release of Damage-Associated Molecular Patterns (DAMPs). This process triggers the death of lung cells by activating a local immune response, with the sensitization of macrophages and monocytes responding to the release of cytokines, along with the involvement of T and B lymphocytes. In cases where the immune response is inefficient, subsequent pyroptosis allows the recognition of DAMPs, prolonging inflammation and leading to an increased secretion of pro-inflammatory cytokines such as IL-6, IFNγ, IP-10, and MCP1. This excess cytokine secretion may trigger a loss of control of the immune system, potentially resulting in a cytokine storm. (24-30.
One significant theory suggests that SARS-CoV-2 infection may initiate GBS through molecular mimicry, a mechanism proposed within the framework of coinfection with SARS-CoV-2. This hypothesis posits that autoimmunity, fueled by molecular mimicry, plays a pivotal role. The virus's spike protein binding to the ACE2 receptor triggers an autoimmune response, leading to the production of antibodies. These antibodies, in turn, target the nerve axolemma, potentially triggering the onset of Guillain-Barré syndrome19,20.
In the autoimmunity hypothesis, autoantibodies attack complexes on glycoproteins surfaces since the Spike protein binds to saccharides and gangliosides of peripheral nerves. However, there is little evidence showing this association. (22,31 In addition, anti-ganglioside antibodies (GD1a/GD1b) are usually found affecting the cranial nerves and are associated with the GBS axonal phenotype; however, the predominance of the demyelinating phenotype and the absence of anti-ganglioside antibodies do not allow us to establish a relationship between the axonal phenotype, COVID-19 and anti-ganglioside antibodies22,32.
In a study on mice infected with SARS-CoV in 2007, an increase in cytokines and chemokines was observed in the cerebrospinal fluid, including interleukins (IL-6, -8) and TNF-α, indicating an inflammatory response at the nervous system level. 33
In the year 2000, a study analyzing five individuals who succumbed to SARS-CoV and five control subjects determined the presence of SARS-CoV RNA and antigen in the brain region. This suggests that the virus directly affects the central nervous system, causing damage to peripheral nerves, thereby indicating a potential mechanism of direct harm. (34
Predisposing factors have been evidenced, such as intestinal dysbiosis associated with COVID-19, which correlates with an imbalance in the immune system. This is explained by the role of the microbiota in regulating the balance of Th17 cells and regulatory T cells. (35 There is also a similarity in the microbiota of patients infected with SARS-CoV-2 and patients with autoimmune diseases such as Systemic Lupus Erythematosus; both exhibit a loss of microbiota biodiversity, an increase in pathobionts, and a decrease in symbionts associated with anti-inflammatory properties. (36
Furthermore, it has been observed that certain medications used during the pandemic to treat COVID-19 could be linked to the development of GBS. A 1998 study revealed that the use of immunosuppressive medications could promote the growth of gastrointestinal infections caused by C. jejuni, potentially inducing Guillain-Barré Syndrome. (37 It is known that this bacterium exhibits cross-reactivity with viral antigens, which could contribute to the development of the mentioned syndrome. (38
Motor symptoms
We found that predominantly 11.11% (n=8) of the patients the first neurological symptom was muscle weakness in the lower limbs compared to what was reported by Meythaler et al., where 60% (n=42) initially presented it in lower limbs and 20% (n=14) in upper limbs. (39 After that study, Cosi et al. similarly, 56% reported initial muscle weakness in the lower limbs, 12% in the upper limbs, and up to 32% in a generalized way. (40 However, in our study, it is noteworthy that only some cases offered specific information about the initial manifestation, such as lower limb weakness, while others provided more general descriptions.
In the same study by Cosi et al., a case of ataxia, and 4.76% of paraparesis40 were reported. In our review, we found only 5.56% (n=4) of cases that presented ataxia, and 6.94% (n=5) abasia, while 5.56% (n=4) reported paraparesis.
Sensory symptoms
Wang et col. reported 32.50% (n=170) with paresthesia, and 30.78% (n=161) with hypoesthesia and numbness. (41 In our review, paresthesia was also described, as being more frequent in lower limbs at 20.83% (n=15), followed by both lower and upper limbs at 6.94% (n=5) and 2.78% (n=2) just in upper limbs. The same authors also reported hypoesthesia, 16.67% (n=12) in both lower and upper limbs, 8.33% (n=6) in lower limbs, and 1.39% (n=1) in upper limbs. (41 However, we reviewed only one patient who presented hyperesthesia in the back, while in the retrospective study, hyperesthesia was not reported.
Back pain was present in 16.66% (n=12) in our review. However, other authors reported that 34.5% (n=87) had back pain, of which 29.9% (n=26) reported lower back pain. (42
Autonomic Dysfunction
In 2020, a study evaluated 118 patients, of which 41.53% (n=49) reported at least one type of autonomic dysfunction. (43 The most common were defecation dysfunction, including constipation (n=26) and diarrhea (n=25), followed by blood pressure abnormalities (n=23). (43 Additionally, urinary retention (n=18) and heart rate abnormalities were found (n=16).43 In our review, only 13.89% (n=10) found at least one type of autonomic alteration; the most frequent being the alteration of urination followed by fecal incontinence. While blood pressure and heart rate abnormalities were found in two patients. Besides, only one patient presented diarrhea. Therefore, we found autonomic dysfunction was less frequent during the COVID-19 pandemic.
Cranial nerves (CN) compromise
In a study conducted in 2014, of 68 patients with GBS, 62.3% (n=38) presented at least one CN compromise, being the most common bulbar paralysis and dysphagia (n=30), followed by facial paralysis (n=28) where only three had a unilateral compromise. (44 The least common CN compromise were hypoglossal (n=6), ophthalmoplegia (n=4), and vestibulocochlear nerve dysfunction (n=1). (44) In our review, cranial nerve involvement was clinically described in 38.89% (n=28) and reported on electromyography in 6.94% (n=5), much lower than before the COVID-19 pandemic. Regarding CN compromise, the most common was bulbar palsy causing dysphagia; In comparison with the findings of our review, the most frequent finding was facial paralysis reported clinically in 38.89% (n=28) and 2.78% (n=2) by electromyography. . (44 It is worth mentioning that, in both cases, bilateral bulbar palsy predominated over unilateral.
The Role of cerebrospinal fluid (CSF) analysis
An important laboratory finding of GBS is albumin-cytological dissociation, representing the increase of CSF albumin in the absence of hypercellularity. (45 However, around 30-50% of patients do not experience significant CSF protein increase during the first week; and 10-30% during the second. (45) Because of these findings, a normal protein count cannot rule out GBS. However, our findings suggest the consistency of this finding along the SGB cases associated with COVID-19. In addition, one patient was reported positive for SARS-CoV-2 by RT-PCR in CSF, but the rest were negative.
Radiological Imaging in the Context of GBS and COVID-19
In the first months of the COVID-19 breakthrough, Wong et al. and Chung et al. reported radiological features associated with COVID-19 pneumonia in X-ray imaging and CT scans. Among the principal findings, ground glass opacity and consolidations are predominantly at the peripheral level, typically involving both lungs. (46,47 Nevertheless, imaging findings between isolated COVID-19 and co-infection with GBS do not differ significantly.
MRI scans have limited diagnostic accuracy for GBS diagnosis but may be useful in atypical presentations. Before 2019, root nerve contrast enhancement was reported as a common MRI finding, especially at the cauda equina and conus medullaris levels in lumbar MRI48-52.
This revision highlights the same MRI characteristics for GBS patients with COVID-19 coinfection. In addition, enhancement of the cervical leptomeningeal level and brainstem were reported (18%), alongside foraminal stenosis (18%).
GBS variants and COVID-19 coinfection
The first case series and observational studies describe a symptomatic resemblance between SARS-CoV-2 infection and GBS. (53 During the outbreak, a review reported Acute Inflammatory Demyelinating Polyneuropathy (AIDP) as the principal variant in 26 patients (59.1%)54. However, before the pandemic, AIDP frequency peaked at 85-90%, followed by Acute Motor Axonal Neuropathy (AMAN) and Acute Motor-Sensitive Axonal Neuropathy (AMSAN) at 5-10%.55,56 However, in our review, both AMAN and AMSAN variants were found in 13.89% and 12.5%, respectively. So, it seems that the frequencies may have increased.
Less common variants like Miller-Fisher Syndrome (MF) or Bickerstaff Encephalitis were also reported.57 Even after the SARS-Cov-2 outbreak, both GBS variants did not experience an increase. Finally, 10 cases were cataloged as non-otherwise specified GBS, probably because of the unavailability of neurophysiological testing.
Nerve conduction studies and GBS
In 2021, a retrospective report of 31 clinical cases found 84% of abnormal F waves (prolonged or absent latency), 65% presented a prolonged distal motor latency at least in one nerve, and a decrease of conduction velocity in 52% of patients.58 Our findings also support the predominance of the absent F wave in most co-infection cases.
In another case-control study published in 2016, it was reported that all evaluated patients presented a prolonged distal motor latency and a reduction in motor amplitude (n=37) in the median, ulnar, and peroneal nerves. (59 Notably, in the first week of illness, motor conduction velocity was decreased in the median and peroneal nerves and preserved in the ulnar nerve. (59 The results found in our review are similar to these findings, as prolonged distal motor latency and decreased motor conduction velocity were the most frequent findings. However, in our findings, ulnar nerve involvement (44.74%) was evident, being the second most frequent after the median nerve (50%).
Conclusions
To our knowledge, this work represents a most complete and comprehensive analysis of COVID-19 and SGB. Our principal results follow. The most common type of GBS-COVID-19 association was acute inflammatory demyelinating polyneuropathy (AIDP). This variant was also frequent before the pandemic, indicating no distinct predominance in SARS-CoV-2 infected patients. The clinical findings in our review revealed that paresthesia predominantly in the lower limbs and hypoesthesia in the upper and lower limbs were the most frequent sensory abnormalities, along with back pain. Regarding motor function, global muscle weakness was the most common alteration.
Similarly, nerve conduction findings in patients during the pandemic were comparable to those observed in patients with GBS before the pandemic, with the most common abnormality being the absence of F waves.
Autonomic abnormalities were less prevalent in patients with SGB-COVID-19 association compared to cases reported before the pandemic, with the most frequent autonomic abnormalities in this review were urinary alterations, which were not as frequent in pre-pandemic cases, whereas defecation alterations were more common.
Regarding cranial nerve involvement, facial paralysis was the most common in our review compared to cases before the pandemic, where bulbar paralysis was more frequent. The only similarity was that bilateral facial paralysis predominated over unilateral paralysis. It is important to note that the prevalence in our review was much lower compared to pre-pandemic SGB cases.
The most frequent characteristics found in cerebrospinal fluid (CSF) analysis were elevated protein levels and albumin-cytological dissociation, which did not differ from cases reported before the pandemic.
Based on magnetic resonance imaging (MRI) reports, the most common characteristic was nerve roots and cranial nerve enhancement. However, additional findings included cervical leptomeningeal and brainstem enhancement (18%) and foraminal stenosis (18%) in lumbar MRI scans.