INTRODUCTION
Orthodontic forces induce physical and chemical responses in periodontal tissues. The mechanical stimulus produces an acute inflammatory reaction within periodontal tissues, which may trigger a series of biological processes that result in bone resorption to allow tooth movement. The reabsorption mechanism induces the release of inflammatory mediators, such as interleukins (IL), tumor necrosis factor (TNF-α), prostaglandins, among others; at the biomolecular level 1.
The use of miniscrews in orthodontic therapy has expanded, due to the stability of the cortical anchorage they provide; especially during space closure, compared to conventional techniques and their easy insertion and removal 1,2. However, one of the main problems is the instability or failure of the temporary anchorage device. Meffert suggested that failure will be accompanied clinically by increased probing depth, pain in the area, and/or radiographic bone loss 3. This process has been named peri-implantitis. Numerous studies on dental implants have reported that an increase in the levels of pro-inflammatory cytokines in the peri-implant crevicular fluid causes peri-implantitis. 4-6 Likewise, some studies report significantly higher levels in patients with implants that fail compared to those in good condition 1,2.
Still the effect of miniscrews at the biomolecular level is not usually evaluated.
The purpose of this study was to determine the levels of cytokines (IL-8, IL-1β, IL-6, IL-10, TNF-α, IL-12p70) simultaneously, in saliva and peri-implant crevicular fluid around miniscrews with and without load during orthodontic treatment, using a Cytometric Bead Array (CBA).
MATERIAL AND METHODS
This descriptive longitudinal study was conducted after the approval of the Ethics Committee (document number R-84-19-15). Informed consent or assent was obtained from the patients and the consent of the parents in case they were minors, according to the institutional protocol, confidentiality mechanisms of clinical data were also contemplated. Eight patients between 14-50 years comprised the study sample. The inclusion criteria were a healthy systemic condition, non-pregnant women, no use of anti-inflammatory drugs one week preceding the start of the study, and no radiographic evidence of periodontal bone loss. The patients required the use of miniscrews (Dewimed- Germany) according to their orthodontic treatment plan making a total sample of 10 miniscrews (Figure 1). Patients received oral hygiene instructions after the placement of orthodontic appliances.
Collection of saliva and peri-implant crevicular fluid samples
Patient samples of the selected areas were carried out according to the following schedule:
T0 (baseline): saliva sample
T1 (24 hours post-insertion) no loading: saliva and peri-implant crevicular fluid.
T2 (1-week post-insertion) no loading: saliva and peri- implant crevicular fluid.
T3 (24 hours post miniscrews loading): saliva and peri-implant crevicular fluid.
In the case of bleeding, it was controlled before taking the sample. Cotton swabs were used to obtain saliva samples and then placed in a microcentrifuge tube. Subsequently, they were centrifuged and stored at a temperature of -20 °C until their analysis (Figure 2).
The peri-implant crevicular fluid was performed using a similar method to the one used by Sari and Uçar (1). The area where the mini-implant was inserted was isolated with cotton rolls, air from the triple syringe, and suction was used to keep the area dry. Endodontic filter paper cones No. 40 were used. The filter paper cone was placed at the bottom of the peri-implant sulcus until a slight resistance was felt, to absorb the peri-implant crevicular fluid, approximately 1 minute per cone. Four cones were used per sampling time, which were collected in the same tube. Then 50 uL of phosphate buffer saline (PBS) was added, and it was centrifuged at 13000 rpm for 1 minute to extract the sample from the paper cones. The samples obtained were then stored at -20 °C until their subsequent analysis (Figure 3). The tubes containing the paper cones were weighed on an analytical balance before and after taking the sample, to calculate the weight of the sample obtained and its corresponding volume.
Cytokine Analysis
The levels of the cytokines IL-8, IL-1β, IL-6, IL-10, TNF-α, and IL-12p70 were evaluated in the samples obtained from saliva and peri-implant crevicular fluid. The inflammatory markers were simultaneously identified by an immunoassay read in a flow cytometer (Fluorescence-Activated Cell Sorter - FACS). The BD Cytometric Bead Array® (CBA) Human Inflammatory Cytokines kit (BD Bioscience, San José, CA, USA) used, allows the simultaneous determination of six cytokines: IL-8, IL-1β, IL-6, IL-10, TNF-α, and IL-
12p70. The protocol followed for this procedure was indicated by the manufacturer (7).
Statistical evaluation
The data were analyzed using the SPSS version 21.0 statistical package. To verify that these followed a normal distribution, the Kolgomorov-Smirnov test was used. Later the analysis of variance test (ANOVA) was used to compare and determine statistical significance (p <0.05). To verify these results, the Scheffé post hoc test was applied.
RESULTS
The levels of inflammatory mediators in the peri- implant crevicular fluid at 24 hrs. post-insertion (T1) of the 10 miniscrews had the following order of mean values: IL-8 (16275.51 pg/mL)>IL-1β (2662.59 pg/ mL)>IL-6 (1871.62 pg/mL)>TNF-α (48.865 pg / mL)>IL-10 (4.66 pg/mL) > IL- 12P70 (0.045 pg/mL). The values found one-week post-insertion (T2) and at 24 hrs. post-loading (T3) are shown in Table 1. In all the samples taken (T1, T2, and T3) IL-12P70 showed the lowest concentration including a value of zero at T2. However, there was an increase at (T3); the previously mentioned hierarchy was maintained. In T2, the values begin to decrease and in T3, after force application, a decrease was observed for IL-8 and IL-10 values while an increase of concentration was found in the rest of the cytokines. The highest values of inflammatory mediators were quantified at 24 hours post-insertion.
Table 1 Peri-implant crevicular fluid
| Time point | Interleukin | Mean | S.D | Minimun | Maximun |
|---|---|---|---|---|---|
| 24h post-insertion (T1) | IL-8 | 16275.510 | 20176.329 | 310.350 | 68030.550 |
| IL-1β | 2662.590 | 2868.450 | 61,85 | 8038.500 | |
| IL-6 | 1971.620 | 4069.860 | 2.300 | 13172.850 | |
| IL-10 | 4.660 | 11.737 | 0.000 | 37.850 | |
| TNF-α | 48.865 | 80.422 | 0.000 | 231.800 | |
| IL-12p70 | 0.045 | 0.142 | 0.000 | 0.450 | |
| 1-week post-insertion (T2) | IL-8 | 14255.160 | 20106.252 | 285.050 | 59872.500 |
| IL-1β | 948.485 | 861.177 | 18.850 | 2480.100 | |
| IL-6 | 539.240 | 796.945 | 2.850 | 2207.350 | |
| IL-10 | 3.940 | 6.272 | 0.000 | 15.550 | |
| TNF-α | 13.520 | 16.046 | 0.000 | 46.100 | |
| IL-12p70 | 0.000 | 0.000 | 0.000 | 0.000 | |
| 24h post-loading (T3) | IL-8 | 11001.715 | 10409.175 | 278.950 | 31667.500 |
| IL-1β | 1787.044 | 1446.809 | 206.300 | 4858.700 | |
| IL-6 | 802.095 | 1265.375 | 0.000 | 3387.799 | |
| IL-10 | 2.375 | 4.255 | 0.000 | 12.800 | |
| TNF-α | 47.910 | 7.317 | 0.000 | 184.450 | |
| IL-12p70 | 0.120 | 0.379 | 0.000 | 1.200 |
Kolgomorov-Smirnov test Values are expressed in pg/mL
Table 2 shows the levels of inflammatory mediators found in saliva at different time points. It was observed that in T0 (baseline) almost all inflammatory mediators were detected with the exception of IL-10 and IL-12P70. Likewise, at T1 there was an increase in the levels of all mediators except IL-10 and IL-12P70, which were not detected. At T2 the levels began to decrease for IL-8, IL-1β, and IL-6, while IL-10, TNF-α, and IL-12P70 were not detected. Finally, at T3 the mediator levels continue to decrease but TNF-α was detected. As in the values found for peri-implant crevicular fluid, the hierarchy of the concentrations was maintained.
Table 2 Saliva
| Time point | Interleukin | Mean | S.D | Minimum | Maximun |
|---|---|---|---|---|---|
| Baseline (T0) | IL-8 | 1167.11 | 1884.158 | 131.05 | 6263.900 |
| IL-1β | 102.600 | 161.322 | 1.650 | 533.900 | |
| IL-6 | 77.250 | 191.662 | 0.000 | 600.700 | |
| IL-10 | 0.000 | 0.000 | 0.000 | 0.000 | |
| TNF-α | 0.410 | 1.116 | 0.000 | 3.550 | |
| IL-12p70 | 0.000 | 0.000 | 0.000 | 0.000 | |
| 24h post-insertion(T1) | IL-8 | 6743.61 | 11468.14 | 18.00 | 30325.90 |
| IL-1β | 681.840 | 1262.489 | 0.800 | 3737.500 | |
| IL-6 | 470.320 | 888.769 | 0.000 | 2157.450 | |
| IL-10 | 0.000 | 0.000 | 0.000 | 0.000 | |
| TNF-α | 20.265 | 64.085 | 0.000 | 202.650 | |
| IL-12p70 | 0.000 | 0.000 | 0.000 | 0.000 | |
| 1-week post-insertion (T2) | IL-8 | 5,012.8 | 11472.3 | 12.05 | 37041.6 |
| IL-1β | 332.06 | 613.920 | 0.000 | 1765.14 | |
| IL-6 | 196.29 | 585.610 | 0.000 | 1862.00 | |
| IL-10 | 0.000 | 0.000 | 0.000 | 0.000 | |
| TNF-α | 0.000 | 0.000 | 0.000 | 0.000 | |
| IL-12p70 | 0.000 | 0.000 | 0.000 | 0.000 | |
| 24h post-loading (T3) | IL-8 | 1993.9 | 2134.62 | 14.90 | 7,341.70 |
| IL-1β | 171.48 | 194.940 | 10.60 | 543.900 | |
| IL-6 | 185.41 | 356.435 | 0.000 | 995.550 | |
| IL-10 | 0.000 | 0.000 | 0.000 | 0.000 | |
| TNF-α | 6.465 | 15.017 | 0.000 | 45.700 | |
| IL-12p70 | 0.000 | 0.000 | 0.000 | 0.000 |
Kolgomorov-Smirnov test
Values are expressed in pg/mL
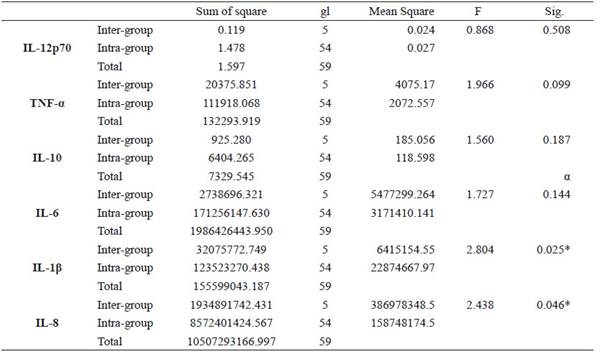
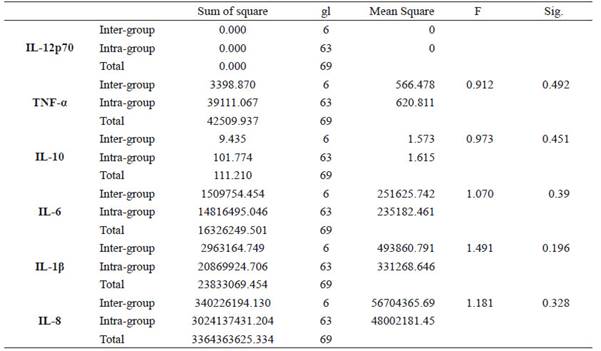
Statistically significant intergroup differences (p <0.05) were found for IL-1β and IL-8 (See Table 3) in the peri-implant crevicular fluid. Subsequently, post hoc tests were carried out to verify these results. The Scheffe test showed that there were no statistically significant differences when performing an intragroup pairwise comparison of the levels of each mediator at a given time. Finally, no statistically significant differences (p <0.05) were found between and within groups for all the inflammatory mediators evaluated in saliva (Table 4).
DISCUSSION
Orthodontic forces induce chemical and physical responses in periodontal tissues. At the onset of orthodontic movement, the mechanical stimulus causes acute inflammation in the periodontal tissues, which can trigger biological processes that result in bone resorption to accommodate tooth movement, and this mechanism, in turn, can induce the release of inflammatory mediators 1.
Control of anchorage is a fundamental concept in orthodontic treatment. Thus, treatment goals should consider the effectiveness of anchorage devices for a more efficient correction of the patient’s dental or skeletal malocclusion 2. Pro-inflammatory cytokines play an important role in bone and root resorption 8. Some studies have evaluated cytokine levels in dental crevicular fluid during orthodontic treatment, showing a significant increase in pro-inflammatory mediators that produce loss or failure of dental implants, but few have reported the role of IL- 8, IL-1β, IL-6, IL-10, TNF-α, and IL-12p70 around orthodontic miniscrews 2. The purpose of this study was to measure the levels of these cytokines present in saliva and peri- implant crevicular fluids of miniscrews with and without loading during orthodontic treatment.
Cytokine levels were measured at the baseline (T0), 24 hours post-insertion (T1), 7 days post-insertion (T2), and 24 hours post miniscrew loading (T3) in saliva and peri-implant crevicular fluid. According to Serra et al., there was no association between age and gender and the increase of enzymatic activity, the reason for which these variables were not considered in our study 9.
Our results demonstrate that the highest concentration of cytokines in the peri-implant crevicular fluid was observed 24 hours after insertion of the miniscrew, with the following hierarchy of mean values: IL-8> IL-1β> IL -6> TNF-α> IL-10> IL-12P70. In T2 the concentrations began to decrease and in T3, although almost all the cytokines experienced an increase (except for IL-8 and IL-3).
On the other hand, almost all inflammatory mediators were detected in saliva except for IL-10 and IL-12P70. In T1, there was an increase in the levels over baseline measurements in saliva samples, while in the crevicular fluid in T2, the concentration began to decrease for IL-8, IL-1β, and IL-6. IL-10, TNF-α, and IL-12P70 were not detected. In T3, although the levels continue to decrease, TNF-α was detected. This increase may be because this mediator affects bone metabolism directly, and in low concentrations, this process has been associated through specific receptors in the bone cell population. Activated monocytes and macrophages synthesize and release these cytokines. Likewise, this could have been caused by chemotactic activities in direct response to the application of a force 10. Another possible cause of the elevation of these levels in T3, could be poor oral hygiene around peri-implant tissues 11. In this study, patients received oral hygiene instructions after the placement of orthodontic appliances.
The only intergroup differences (p <0.05) were found for IL-1β and IL-8 in peri-implant crevicular fluid, but not in saliva. IL-1β appears to be a potent inducer of bone resorption and inhibitor of bone formation 1. The multiple activities of IL-8 indicate that this cytokine plays an important role as a mediator of the inflammatory response, has a multifunctional role in the pathogenesis of the periodontal disease, and orthodontic forces can produce changes in their concentration 1,11. Because periodontal tissues are remodeled on both, tension and compression sides, the immediate increase in IL-8 levels after the application of mechanical forces may be a sign of neutrophil reaction in the area. After the acute response, stimulation of this cytokine can continue on the tension side. Thus, under the influence of mechanical forces, the tension sides would indirectly contribute to the production of this cytokine. 12).
Sari and Uçar examined the levels of interleukin 1β around miniscrews used as anchoring units for the distal movement of canines, with an immunoassay (ELISA). The results showed that in the treatment group the values increased significantly 24 and 48 hours after insertion while the levels in the control group and the implant, the group did not vary significantly during the experimental period. It was shown that the miniscrews did not demonstrate increased 1L-1β levels during tooth movement 1. However, in this study there were no samples taken before or after the insertion of the mini-implant without loading, so it is unknown whether the insertion of the mini-implant produced a greater increase in the levels of this interleukin compared to the values found after loading application after two weeks.
Another study conducted by Monga et al. analyzed IL-1β levels at the peri-implant level in 11 patients. 13 In this case, the miniscrews were loaded 3 weeks post-insertion, and samples were taken 4 hours post-insertion, after 3-weeks without loading and 21,72,120,180, and 300-days post-loading. They found that the levels of this cytokine were significantly higher in T1 (4 hours post-insertion). Another peak was observed in T4 (24 hours post-loading), however, there was a significant decrease in T5 (72 days later). These results are similar to those found in this research.
Hamamci et al., reported that the increase in cytokine levels, especially IL-8, around miniscrews and canines were quite similar, during the first hours as a result of an acute inflammatory reaction 2. Therefore, since the mediators are effective in the early stages of inflammation, it is important to determine the magnitude of the initial force to be used 2. In our study, IL-8 was one of the cytokines whose values were statistically significant; however, these decreased in T3 (24 hours post-loading) which is the opposite of what these authors found. It should be considered that we loaded the miniscrews one week after insertion, while in the research of Hamamci et al. the loading was 2 weeks after insertion, and no samples were taken 24 hours after it which is why we cannot assert that the loading produced more inflammation than the placement of the device per se.
Recently, He et al., carried out a review to investigate the potentially essential factors in the inflammatory response around the peri-miniscrew implant and explore the signaling pathways involved 14. The literature search revealed that IL-1β and IL-6 are the critical inflammation factors in the signaling pathways inducing the inflammatory reaction surrounding implants. Besides, cellular adhesion molecules (CAM-1) were also regulated by MMP-9 and IL-17. So, there are considerable potential factors involving regulating inflammatory biomarkers on downstream signaling pathways in peri-miniscrew implant crevicular fluid that should be evaluated in future studies 14.
Finally, our small sample size could have influenced the interpretation of our results that is why they must be interpreted with caution since they cannot be fully contrasted with previous ones. First, because the time points were not the same and in most of the publications that precede this study, no post-insertion samples were taken from the miniscrew neither in saliva nor the crevicular fluid. Secondly, because the method by which the samples were analyzed was not the same; previous studies used the classical ELISA immunoassay whereas in this study samples were processed with a system known as BD Cytometric Bead Array (CBA) 7).
In the enzyme-linked immunosorbent assay (ELISA), a typical double antibody sandwich, one antibody binds to a plate with capture antigens and immune specificity, while another antibody bound to an enzyme provides a detection and amplification factor. This scope allows precision and sensitivity in the detection of the antigen, cytokine of interest. Some of the advantages of this procedure are that kits to measure these cytokines are available on the market, in addition to being highly quantitative and reproducible. At the same time, it has disadvantages such as: being highly dependent on the quality of the antibody, the manufacturer of the kit, the experience of the operator. It allows the reading of a single cytokine at a time, it cannot perform the reading in very small volumes, so there is difficulty in comparing the levels of two cytokines measured by two different ELISA arrangements. Finally, another limiting factor is the range of dynamics (range in which there is a linear relationship between the concentration of cytokines and the adsorption reading) which is relatively low compared to other technologies. This is why concentrations above this range must be diluted and this not only reduces the concentration of the cytokines to be measured but also reduces the concentration of an inhibitor or binding protein 14.
On the other hand, multiple assays have been developed from ELISA to measure multiple cytokines simultaneously and are available in different formats based on the use of flow cytometry, chemiluminescence, or electrochemiluminescence technology. In this case, a flow cytometry microarray also known as a bead- based multiplex assay was used, which probably represents the most common formation at this time. Each bead is coated with a specific capture antibody and the fluorescent antibodies bind to a capture antibody- specific cytokine complex. Thus, multiple cytokines present in a biological fluid can be recognized and measured by the difference in the sets of beads, with or without fluorogenic emissions detected using flow cytometric analysis. Currently, some kits can measure up to 25 cytokines simultaneously. Compared to ELISA, this type of microarray has several advantages that include: high throughput multiple analysis, minimum required volume, efficiency in terms of time and cost, ability to evaluate the levels of a given inflammatory molecule in the context of others, the ability to perform repeated measurements of the same panel of cytokines in the same subjects under an experimental setup condition and the reliability of detecting different proteins across a dynamic range of concentrations 13, few studies use CBA system for sample processing, so more studies are required in this line to make reliable comparisons of the results obtained.
The miniscrew loading did not generate an increase of the concentrations of the cytokines greater than the effect caused by the insertion of the mini-implant per se. The highest levels of inflammatory mediators were found 24 hours post-insertion of the miniscrew.
We suggest researchers to use this laboratory analysis in future studies and increase the sample size to find an association between biomarkers levels and miniscrews failure in order to improve the clinical performance of these devices.