Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Gastroenterología del Perú
versión impresa ISSN 1022-5129
Rev. gastroenterol. Perú v.33 n.2 Lima abr./jun. 2013
ARTÍCULO DE REVISIÓN
The clinical importance of serrated lesions of the colorectum
La importancia clínica de las lesiones serradas del colon y recto
Carol Burke 1a
1 Director, Center for Colon Polyps and Cancer Prevention, Department of Gastroenterology and Hepatology, Cleveland Clinic, Cleveland, Ohio, EE UU.
a MD, FACG, FASGE, FACP
ABSTRACT
Colorectal cancer (CRC) is the third most common cancer in the world. Fortunately, it is also proven to be one of the most preventable cancers, in large part due to the utilization of CRC screening. Historically, it was believed that the adenomatous polyp was the only precursor to carcinoma of the colorectum. Within the last decade, it has been shown that approximately 20-30% of sporadic colon cancers arise through a distinct molecular pathway called CpG Island Methylation (CIMP) which is due to widespread DNA methylation. There is strong evidence that serrated polyps are the precursor lesions for colon cancers arising through the CIMP pathway.
Key words: Colorectal neoplasms; Colon; Rectum (source: MeSH NLM).
RESUMEN
Cáncer Colorectal (CRC) es el tercer cáncer más común en el mundo (1). Afortunadamente también se ha probado que es el cáncer que más se puede prevenir, en gran parte debido al "screening" del CRC. Históricamente, se creía que el pólipo adenomatoso era el único precursor del carcinoma de colon y recto. En la última década se ha demostrado que aproximadamente el 20 al 30% de los cánceres colónicos esporádicos se derivan de una vía molecular diferente llamada Metilación de las Islas CpG (CIMP) que es debida a una extensión de la metilación del DNA.Actualmente hay una fuerte evidencia que los pólipos serrados son las lesiones precursoras de cáncer de colon surgiendo desde la vía CIMP.
Palabras clave: Cáncer colorrectal; Colon; Recto (fuente: DeCS BIREME).
Colorectal cancer (CRC) is the third most common cancer in the world (1). Fortunately, it is also proven to be one of the most preventable cancers, in large part due to the utilization of CRC screening. Historically, it was believed that the adenomatous polyp was the only precursor to carcinoma of the colorectum.
Chromosomal instability (CIN), characterized by an accumulation of progressive genetic alterations in tumor suppressor genes and oncogenes, results in the transformation of an adenoma into a microsatellite stable (MSS) CRC (2). It is now recognized that CIN only accounts for the genesis of approximately 70-80% of colon cancers. Within the last decade, it has been shown that approximately 20-30% of sporadic colon cancers arise through a distinct molecular pathway called CpG Island Methylation (CIMP) which is due to widespread DNA methylation (3). Methylation of the CpG Islands in the promoter area of a gene halts gene transcription and induces gene silencing. There is strong evidence that serrated polyps are the precursor lesions for colon cancers arising through the CIMP pathway.
Serrated colorectal lesions have long been described. In thelast3decadesanexpandedspectrumofserratedlesions has been characterized. The World Health Organization classification of serrated lesions of the colorectum4 is: Hyperplastic polyp (HP); Sessile serrated adenoma/polyp (SSA/P) without or with cytological dysplasia (SSA-CD) and Traditional serrated adenoma (TSA)
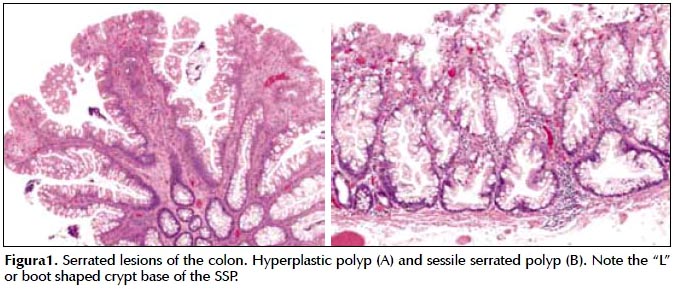
Histologically, serrated lesions have a saw-tooth or serrated configuration of the crypt epithelium (Figura 1). Hyperplastic polyps are the most common and believed not to impart an increased risk of cancer in individuals who harbor them. Sessile serrated polyps (SSP), also known as sessile serrated adenomas, have been shown to be associated with inactivating mutations in the BRAF oncogene, progressive DNA methylation and ultimately promoter methylation of MLH1 which is believed to be a transformative event from an SSP to a microsatellite instable (MSI) cancer. Traditional serrated adenomas (TSA) are the least common of the serrated neoplasms and account for < 1% serrated lesions (4).
Nearly 20 years ago, data demonstrated that colonoscopy and removal of adenomas was associated with a decrease in the incidence of CRC (5). Most recently this observation has also been extended to a reduction of CRC mortality by up to 53% (6). Unfortunately, it has become clear over the last decade that there is variable protection from the use of colonoscopy on CRC incidence and mortality (7-12). Interval cancers, which are cancers which develop after a colonoscopy and before the next recommended interval, are of increasing recognition and concern. Interval cancers have been shown to occur in up to 9% of individuals with CRC who have undergone colonoscopy in the preceding 3 years (12). While there is strong data that the use of colonoscopy has been associated with a decrease in overall and left sided CRC mortality, data have shown a lesser benefit of colonoscopy in the reduction of CRC mortality in the proximal colon (8,9).
A variety of possibilities have been suggested to account for the variable protection from colonoscopy and interval cancers. Factors directly associated with the endoscopist have been determined. These include the specialty of the provider, in particular procedures done by a non-gastroenterologist, or by an endoscopist with low rates of adenoma detection, polypectomy or cecal intubation (10,12). Other factors include the technical limitations of the exam, missed or insufficient resection of lesions, inadequate bowel preparation, and the varying biologic behavior of lesions. The factors most likely to be contributing to interval cancer is the variability in the detection of SSP by the endoscopist and inadequate resection those lesions.
Potential link between serrated lesions and interval cancers: molecular biology
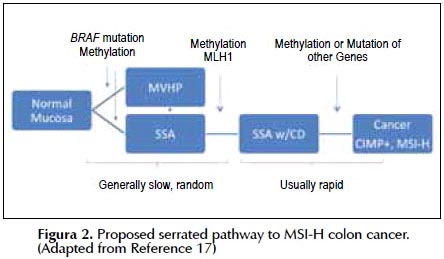
Sawhney et al searched their cancer database for interval cancers defined as cancers developing within 5 years of a complete colonoscopy and matched them by age and gender to subjects who had a CRC detected on their first recorded colonoscopy. 5% of cancers were interval cancers. Interval cancers were four times as likely as non-interval cancers to have MSI (13). In a follow up study they showed that the interval cancers were more likely to be proximal and associated with CIMP (14). Numerous studies have confirmed that the majority of serrated cancers have CIMP 90%, MSI 50%, and BRAF mutations, 82% which is a similar molecular fingerprint as noted in SSP: CIMP 76%, MSI 72%, and BRAF mutation, 83%. These features are distinct and substantially different than the molecular alterations found in adenomas (15). The clinical association between CRC and serrated lesions of the colon was first noted in 1996 in a patient with hyperplastic polyposis, now called serrated polyposis syndrome (16). It is now established that there is a serrated polyp-cancer pathway. It is hypothesized that either normal mucosa or a microvesicular hyperplastic polyp transforms through an activating mutation in BRAF, further methylation, CIMP, within promoter regions of genes, to an SSA (Figura 2). Cytologic dysplasia within an SSA occurs when the DNA mismatch repair enzyme, MLH1, becomes methylated. It is believed a rapid transformation occurs of the SSA-CD into an MSI-H carcinoma (17).
Prevalence and clinical characteristics
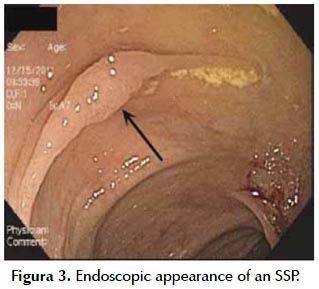
Hiperplastic Polyps (HPs) account for the majority of serrated lesions of the colorectum. HPsare predominantly small, white polyps located in the rectosigmoid. SSP are less common than HP and the prevalence varies according to the ascertainment, be it the lesion versus the patient, the target serrated lesion studied, the use of pathology files versus a colonoscopy database and type of mucosal imaging utilized (white light versus chromoendoscopy or narrow band imaging). In one large study of over 7000 screening colonoscopies done by 13 endoscopists, the prevalence of adenomas was 22%, HP 12% and SSP 0.6% (18). Another study in over 3000 screening colonoscopies from 66 endoscopists demonstrated the SSP detection rate was 2% (19). The endoscopic appearance of an SSP is subtle. They are often the same color as the surrounding mucosa, can be covered with a layer of mucus and have a tendency to look like a prominent fold. One study assessing 7 endoscopic features of SSP found that nearly 50% of SSP express a mean of 2.4 features (20). The prevalence of the characteristic features in the study included a mucus cap (64%), a rim of debris or bubbles (52%), a nodular surface or abnormal fold contour (30-37%), and obscuration of surface blood vessels (32%) (Figura 3). SSPs are usually bigger than adenomas and multiple studies confirm that 50% are > 10 mm (20,21). Less is known of the rare lesion, the TSA, which is usually left sided, more polyploidy in appearance and comprises < 0.5% of all polyps.
Risk to the individual with SSA
The current target of screening and surveillance colonoscopy is the detection and prevention of metachronous advanced conventional neoplasia (AN). Conventional neoplasia refers to adenomatous lesions (tubular, tubulovillous [TVA], and villous [VA]). Advanced neoplasms are adenomas that are one centimeter or greater in size, harbor any villous component (TVA or VA), high grade dysplasia or invasive adenocarcinoma. Little is known about the risk of metachronous lesions in individuals with serrated colon polyps. Most of the current evidence relates to the risk of synchronous colorectal lesions in patients with serrated polyps. Individuals who harbor serrated neoplasms are at in increased risk of synchronous serrated lesions as well as AN (21-25). Li et al found that both right and left sided, large serrated polyps are associated with a 3 fold risk of synchronous AN (22). Schreiner et al. studied over 3000 patients undergoing screening colonoscopy (23). 8% had ≥ 1 proximal HP or SSP. Patients with either a proximal or large HP or SSP were found to be at increased risk of synchronous AN versus those without those lesions. Vu et al compared the phenotypic expression of polyps found on colonoscopy in 3 cohorts of individuals, those with only SSA (N=180), those with only conventional adenomas (N= 173) and those with SSA and conventional adenomas (N=80) (21). The data demonstrated that individuals who co-express SSP and conventional adenomas have significantly more numerous, larger, SSPs and conventional adenomas and more pathologically advanced conventional adenomas than individuals with only SSA or conventional adenomas. Synchronous CRCs were found exclusively in the cohorts with SSA.
Variability in recognition and diagnosis of SSP
Recent data shows a significant variability in the ability of an endoscopist to detect an SSP. Kahi et al. found a 3 fold difference in adenoma detection rate and 18 fold variability in the detection of at least one proximal serrated polyp (26). Data from Hetzel et al. showed a 7 fold difference in SSP detection rate while the variability in adenoma detection was less than a 3 fold difference (18). They also showed that SSP detection rates increased over time; being 0.6% in 2006 and increasing to 1.1% of exams in 2008. This may be due to an increasing awareness of the clinical importance of SSP by the endoscopist or even increasing ability to diagnose these lesions by the pathologist.
In addition to the variability in the endoscopic detection of SSP, it has been shown that there is substantial variability in the pathologic diagnosis of these lesions. In one study there was less than a 2 fold difference observed in the pathologic diagnosis of adenomas and HPs while the variability in the diagnosis of SSP was shown to be 13 fold (18). Until recently, the lack of consensus on the definition and nomenclature of serrated polyps has created considerable confusion in the accurate histological diagnosis of SSP. Previously, even within gastrointestinal pathologists, only moderate concordance was shown when making the distinction between serrated colorectal polyps (27). Recent studies have shown that the reproducibility of diagnosis of serrated polyps improves when standardized diagnostic criteria adopted by consensus are applied (28). A recently published consensus document on serrated lesions of the colon has recommended that the presence of at least one, unequivocal architecturally distorted, dilated, and / or laterally branched crypt, is sufficient for a diagnosis of SSP (29). Differentiating SSP from HPs is challenging and may be due, in part, to either insufficient tissue or poor specimen orientation. Since one of the important histological features of an SSP is present at the crypt base, proper orientation of the specimen seems important. A recent prospective study compared "usual" specimen handling to a modified protocol where the polyp specimen was flattened at the time of removal and placed into a small envelop before sending to the lab. Once in the pathology laboratory, the polyps were cut into sections after processing rather than before. In that study a substantial increase in the diagnosis of SSP (76% vs. 42%), and decrease in the diagnosis of HP (8% vs. 29.5%) was noted in the modified versus "usual" specimen handling arm (30).
It has been recommended that all serrated lesions greater than 5 mm in size in the recto-sigmoid and all serrated lesions proximal to the sigmoid colon be removed completely (29). It has been believed for some time that serrated polyps were less likely to be completely eradicated than their adenomatous counterparts. One recent study confirmed this belief. In a prospective study by Pohl et al, biopsies from the margins of polyps were performed after snare cautery polypectomy for complete eradication was done. It was found that 10% of polyps were incompletely resected and was significantly higher for 10–20 mm (17%) than 5-9 mm (7%) polyps and SSP (31%) versus conventional adenomas (7%). Additionally the rate of incomplete resection was found to vary between endoscopists (31).
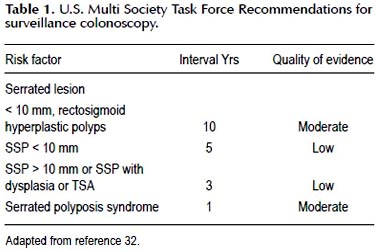
Recently recommendations for post polypectomy surveillance colonoscopy have been updated by the United States Multi-Society Task Force on colorectal cancer (32). It is recommended that individuals with serrated neoplasms including SSP and TSA undergo surveillance based upon the size, histology of serrated lesion and presence of dysplasia (Table 1). As opposed to the strong evidence supporting the recommended surveillance intervals for individuals with adenomatous polyps, the evidence to support the recommendations for serrated lesions is low to moderate. One small cohort study found that patients with a proximal serrated polyp were more likely (17%) than those without a proximal serrated polyp (10%) to have advanced neoplasia on surveillance colonoscopy (23). In another study which identified polyps from pathology archives and assessed the clinical follow up found the incidence of CRC was higher in the SSP patients (12.5%) than in patients with HP (2%) or adenomatous polyps (2%) (33).
From these data it appears that individuals with SSP appear to be group at increased risk, which needs to be accounted for when calculating post-polypectomy surveillance intervals.
There is no doubt that a serrated polyp -cancer pathway exists. The genetic and molecular basis of serrated lesions is established and distinct from the adenoma-carcinoma pathway. Serrated neoplasms are the precursors of approximately 25% of sporadic colon cancers and likely contribute to the occurrence of interval colon cancer. Reducing the substantial variability in endoscopist recognition and pathologist diagnosis of SSP should be targets of quality improvement. Research into the most effective modalities to enhance their recognition, and ensure adequacy of complete resection is warranted. Individuals with SSP are at high risk of synchronous AN and metachronous AN. More data to support appropriate intervals for post-polypectomy surveillance colonoscopy are needed.
REFERENCES
1. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366-78.
2. Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;138(6):2059-72.
3. Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8(12):686-700.
4. Snover D, Ahnen DJ, Burt RW, et al. Serrated polyps of the colon and rectum and serrated ("hyperplastic") polyposis. In: Bozman FT, Carneiro F, Hruban RH, et al, editors. WHO classification of tumours. Pathology and genetics. Tumours of the digestive system. 4th edition. Berlin: Springer-Verlag; 2010.
5. Winawer SJ, Zauber AG, Ho MN, OBrien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27);1977-81.
6. Zauber AG, Winawer SJ, OBrien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-96.
7. Robertson DJ, Greenberg ER, Beach M, Sandler RS, Ahnen D, Haile RW, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129(1):34-41.
8. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1-8.
9. Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139(4):1128-37.
10. Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362(19):1795-803.
11. Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastro Hepatology. 2006;4(10):1259-64.
12. Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140(1):65-72.
13. Sawhney MS, Farrar WD, Gudiseva S, Nelson DB, Lederle FA, Rector TS, et al. Microsatellite Instability in Interval colon cancers. Gastroenterology 2006;131;131(6):1700-5.
14. Arain MA, Sawhney M, Sheikh S, Anway R, Thyagarajan B, Bond JH, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterology. 2010;105(5):1189-95.
15. OBrien MJ, Yang S, Mack C, Xu H, Huang CS, Mulcahy E, et al. Comparison of microsatellite instability, CpG Island Methylation phenotype, BRAF and Kras status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30(12):1491-501.
16. Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110(3):748-55.
17. Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42(1):1-10.
18. Hetzel JT, Huang CS, Coukos JA, Omstead K, Cerda SR, Yang S, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterology. 2010;105(12):2656-64.
19. Sanaka M, Gohel T, Podugu A, Kiran PR, Thota PN, Lopez R, et al. Quality Indicators to enhance adenoma detection rate: should there be reconsideration of the current standard?. Gastrointest Endosc. 2011:73:AB138.
20. Tadepalli US, Feihel D, Miller KM, Itzkowitz SH, Freedman JS, Kornacki S, et al. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video). Gastroinest Endosc. 2011;74(6):1360-8.
21. Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum. 2011;54(10):1216-23.
22. Li D, Jin C, McCulloch C, Kakar S, Berger BM, Imperiale TF, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol. 2009;104(3):695-702.
23. S chreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010;139(5):1497-502.
24. Gurudu SR, Heigh RI, De Petris G, Heigh EG, Leighton JA, Pasha SF, et al. Sessile serrated adenomas: Demographic, endoscopic and pathologic characteristics. World J Gastroenterol. 2010;16(27):3402-5.
25. Pai R, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in non-syndromic patients. Histopathology. 2010;56(5):581-8.
26. Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastro Hepatology. 2010;9(1):42-6.
27. Wong NA, Hunt LP, Novelli MR, Shepherd NA, Warren BF. Observer agreement in the diagnosis of serrated polyps of the large bowel. Histopathology. 2009;55(1):63-6.
28. Ensari A, Bilezikçi B, Carneiro F, Doğusoy GB, Driessen A, Dursun A, et al. Serrated polyps of the colon: how reproducible is their classification? Virchows Arch. 2012;461(5):495-504.
29. Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107(9):1315-29.
30. Morales SJ, Kolb JM, Bodian CA, Kornacki S, Rouse RV, Petras RE, et al. Modifications to serrated polyp handling after polypectomy improve histopathologic section quality and raise the diagnostic rate for advanced lesions. Gastrointest Endosc. 2013;77(3):AB558.
31. Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, et al. Incomplete Polyp Resection During Colonoscopy-Results of the Complete Adenoma Resection (CARE) Study. Gastroenterology. 2013;144(1):74-80.
32. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-57.
33. Lu FI, van Niekerk de W, Owen D, Tha SP, Turbin DA, Webber DL. Longitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinoma. Am J Surg Pathol. 2010;34(7):927-34.
Citar como: Burke C. The clinical importance of serrated lesions of the colorectum. Rev Gatroenterol Peru. 2013;33(2):147-51.
Correspondence:
Carol Burke MD, FACG, FASGE, FACP
Director, Center for Colon Polyps and Cancer Prevention
Department of Gastroenterology and Hepatology Cleveland Clinic
Desk A 30, 9500 Euclid Avenue Cleveland, Ohio 44195
E-mail: burkec1@ccf.org
Recibido: 10/06/2013
Aprobado: 24/06/2013













