Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Gastroenterología del Perú
Print version ISSN 1022-5129
Rev. gastroenterol. Perú vol.34 no.2 Lima Apr. 2014
Artículo de revisión
Endoscopic ultrasound hemostasis techniques
Técnicas de hemostasia por ultrasonido endoscópico
Everson L.A. Artifon1a,2b, Dayse P.S. Aparicio3c, Jose P. Otoch3f, Paulo B. Carvalho3c, Fernando P. Marson3c, Kaie Fernandes3c, Asadur J. Tchekmedyian4d,5e
1 Servicio de Endoscopía, Hospital Ana Costa. Santos. San Pablo, Brasil.
2 Sector de Endoscopia Biliopancreática (CPRE), Servicio de Endoscopía, Hospital de Clínicas de la Facultad de Medicina, Universidad de San Pablo. San Pablo, Brasil.
3 Facultad de Medicina, Universidad de San Pablo. San Pablo, Brasil.
4 Hospital Pasteur, Ministerio de Salud Pública. Montevideo, Uruguay.
5 Sociedad de Gastroenterología del Uruguay. Montevideo, Uruguay.
a Jefe del Servicio, b Coordinador, c Alumnos de post grado, d Gastroenterólogo, e Vice-Presidente, fMédico asistente.
ABSTRACT
Since its development, endoscopic ultrasound (EUS) has evolved from a simple diagnostic technique to an important therapeutic tool for interventional endoscopy. EUS analysis provides real-time imaging of most major thoracic and abdominal vessels, and the possibility to use needle puncture with a curved linear array echoendoscope as a vascular intervention. In this review, we describe the endoscopic ultrasound approach to vascular therapy outside of the gastrointestinal wall.
Key words: Gastrointestinal hemorrhage; Endoscopic ultrasound; Endoscopy (source: MeSH NLM).
RESUMEN
Desde su introducción, la ultrasonografía endoscópica (USE) evolucionó de una técnica diagnóstica a un procedimiento terapéutico. La USE proporciona una imagen en tiempo real de la mayoría de los grandes vasos torácicos y abdominales, brindando la posibilidad de utilizar la aguja de punción a través del ecoendoscopio como una intervención vascular. En esta revisión, describimos la intervención vascular por fuera de la pared gastrointestinal mediante ecoendoscopia.
Palabras clave: Hemorragia gastrointestinal; Ecoendoscopía; Endoscopía (fuente: DeCS BIREME).
INTRODUCTION
Since its development, endoscopic ultrasound (EUS) has evolved from a simple diagnostic technique to an important therapeutic tool for interventional endoscopy. EUS analysis provides real-time imaging of most major thoracic and abdominal vessels. With the potential to use needle puncture with a curved linear array echoendoscope as a vascular intervention. Thus, EUS may offer an attractive approach to vascular therapy outside the gastrointestinal wall (1).
Selective angiographic embolization in recurrent gastrointestinal bleeding has been a well-established therapy performed by interventional radiologists (2,3).
The EUS approach may have advantages in terms of optimally targeting vessels, as compared to more conventional vascular approaches via the femoral, jugular, or subclavian arteries. If used to its full potential, EUS could be the optimal combination of interventional endoscopy and minimally invasive procedures.
Gastrointestinal bleeding represents one of the biggest challenges in therapeutic endoscopy. In approximately 10% to 15% of cases, conventional endoscopic therapy is insufficient and leads to early recurrence of bleeding (4). In these cases, EUS-guided sclerotherapy and embolization representpossible new therapeutic approach.
Some studies have reported EUS hemostasis from nonvariceal to esophageal or gastric variceal gastrointestinal bleeding. The aim of this chapter is to synthetize the current literature in EUS hemostasis such that the reader appreciates the importance and implications of new modalities in therapeutic endoscopy. The following three major areas of EUS hemostasis will be reviewed:
EUS hemostasis of nonvariceal bleeding.
EUS hemostasis of variceal bleeding.
EUS hemostasis of pseudoaneurysms.
1. Eus hemostasis of nonvariceal gastrointestinal bleeding
Several endoscopic therapies are currently in use for gastrointestinal bleeding including adrenaline injection, coagulant current probes, and mechanical approaches using clips or band ligation (5-12). However, unsuccessful bleeding hemostasis and recurrent bleeding can occur in up to 20% of patients (13–15) after initial therapy, which may require repeated endoscopies, interventional radiographic therapy, surgical vessel ligation, or even surgical resection of the affected gastrointestinal segment. EUS-guided therapy may have advantages over standard therapies, because it is highly sensitive for the detection of vascular structures in the gastrointestinal tract wall, particularly in the absence of an endoscopically visible lesion.
EUS hemostasis of nonvariceal upper GI bleeding has been described for peptic ulcer disease, bleeding tumors, and Dieulafoys lesions. In an animal study, Elmunzer et al. created an artificial gastric arterial bleeding model (submucosal surgical placement of gastroepiploic vascular bundle). After echoendoscopic visualization of the submucosal artery, EUS-guided dilute epinephrine injection or contact thermal coagulation therapy were delivered directly to the vessel. The procedure was successful in both cases treated with epinephrine injection and in two out of four treated with contact thermal coagulation (16).
Levy et al. first reported the use of EUS therapy in the management of refractory bleeding. EUS-guided angiotherapy was performed for the management of severe rebleeding after conventional endoscopic or radiologic interventions, and in one patient with chronic anemia in the absence of overt bleeding. Out of five patients, four presented with severe bleeding. They received an average of 18 units of packed red blood cells, and had failed other conventional approaches to control the bleeding. EUS-guided angiotherapy with 99% alcohol or cyanoacrylate injection was performed and beeding was controlled in all of the cases without any complications (17).
Other case reports and small case studies have been reported in literature (18-20). Gonzalez et al. described an EUS-guided injection of cyanoacrylate in a side branch of the gastroduodenal artery, and a successful 19-gauge injection therapy of two Dieulafoy lesions with no recurrence of bleeding in the follow-up period (18). In a small study, Fockens et al. reported EUS-hemostasis of three patients with Dieulafoy lesions using a sector scanner EUS with the aid of an endoscopic picture (19). EUS-guided injection of epinephrine/polidocanol was performed, and all of the procedures were successful without complications, with a rebleeding episode in only one patient. In a case report with the use of Doppler EUS, Ribeiro et al. detected a residual artery five months after local therapy of a Dieulafoys lesion with rubber band ligation. Subsequently, thermal contact therapy followed by EUS-guided injection of absolute alcohol successfully stopped the artery blood flow based on the Doppler EUS analysis (20).
In review of the limited literature in the subject, EUS hemostasis of nonvariceal bleeding appears to be safe and effective in selected patients with lesions potentially refractory to standard endoscopic or angiographic techniques.
2. Eus hemostasis of variceal bleeding
2.1 Esophageal variceal bleeding
Endoscopic band ligation is currently the standard therapy for primary or secondary eradication of esophageal varices, whereas the injection of sclerosants has been used for rescue therapy (21-23). Although eradication is achieved in most of the patients, variceal recurrence is found in 15% to 65% (24,25). This may correlate with failure to treat perforating and collateral vessels that feed the esophageal varices (26,27). Indeed, Krige et al. found a correlation between the presence of collateral vessels and the number of endoscopic sclerotherapy sessions required to achieve eradication of esophageal varices (28). Following the same principle, EUS has become a valuable tool for the diagnosis, treatment planning, evaluation of treatment success, and estimation of recurrent bleeding potential of esophageal varices.
The first report of EUS hemostasis of esophageal varices with sclerotherapy was made by Lahoti et al., who described a small case series of 5 patients with the use of sclerosant agent directed at the perforating vessel until flow was completely impeded. To achieve varix obliteration, 2.2 sessions per patient were needed, and no recurrent bleeding was reported after a mean 15 months follow-up (29). In a randomized controlled trial, Andrade de Paulo et al. compared endoscopic sclerotherapy and EUS-guided sclerotherapy of esophageal collateral veins. They hypothesized that obliteration of collateral veins would reduce the risk of recurrent bleeding of esophageal varices. Between these two groups, there was no difference in the number of sessions required to achieve obliteration or in rebleeding rates: however, collateral veins were undetected in any patient of the EUS-guided therapy group. Accordingly, the presence of collateral vessels at the end of the intervention was associated with variceal bleeding (30).
EUS hemostasis of esophageal varices, especially when directed to collateral vessels, seems to be feasible and effective; however, further studies are needed to determine if this therapy will be accepted as a standard of care in this cohort of patients.
2.2 Gastric variceal bleeding
Gastric varices are a common complication of portal hypertension, which may be present in up to 20% of these patients (31). Although gastric variceal bleeding is less common than esophageal, it usually is more severe and results in higher mortality. Standard therapies for esophageal variceal bleeding, such as band ligation or sclerotherapy, are not recommended for gastric variceal bleeding due to high rebleeding rates (31,32). After its first description by Soehendra et al.(33), the direct endoscopic injection of cyanoacrylate is widely considered to be the therapy of choice for the management of gastric variceal bleeding (34). Nevertheless, transjugular intrahepatic portosystemic shunt remains the first-line therapy in many centers, mainly because cyanoacrylate is an offlabel use and due to the risk of serious adverse events from its embolization (35).
It is well described that embolization is the major complication of cyanoacrylate therapy for gastric variceal bleeding, including reports of cerebral embolism (36). Regarding this complication, alternative therapies have been studied, including EUS hemostasis with fine needle injection of cyanoacrylate, coils, or the association of both (37,38) .
EUS hemostasis of gastric varices with cyanoacrylate injection has been reported and has the advantage of EUS visualization of the injection into the variceal lumen. EUS visualization of the perforating feeding vein enables this vessel obliteration and Doppler analysis afterwards. This EUS-guided approach allows selective injection into the perforating veins. At this location, it can be obstructed with a small amount of cyanoacrylate, which theoretically may result in less adverse events of embolization (39). In a comparative study, Lee et al. reported that EUS monitoring of gastric varix obliteration after cyanoacrylate injection resulted in less recurrent bleeding than with the standard endoscopic injection(40). Romero-Castro et al. described a small case series of 5 patients, in which EUS-guided injection of cyanoacrylate was performed into the perforating feeding veins. They injected a cyanoacrylate-lipiodol mixture in gastric varices with 22-gauge needles under EUS-guidance. All of the procedures were successful in eradicating the gastric varices, without recurrent bleeding or other complications during the study follow-up. However, they report that the most difficult and time-consuming part of injecting the perforating vein of gastric varices is to precisely identify the veinf. Due to the fact that the perforating vein can be an afferent or efferent vessel, they had to inject contrast to determine the directional flow of the varix, which is time and labor intensive (41).
In order to avoid cyanoacrylate embolization, some advanced endoscopists advocate the use of stainless steel coils that can be introduced into gastric varices under EUS guidance (42,43), a known hemostatic therapy with interventional radiology (44-46). In a small case series, Levy et al. reported a successful EUS-guided 22-gauge needle delivery of microcoils in three patients that presented with acute bleeding from ectopic coledochojejunal anastomotic varices (42). Romero-Castro et al. reported a small case series of patients with severe gastric varices treated with EUS-guided coil embolization. They inserted coils into the perforating veins in order to block the blood flow. The varices were eradicated in three out of four patients, and no complications occurred in the successfully treated patients during five months of follow-up (43).
In a multicenter study, Romero-Castro et al. compared EUS-guided coil and cyanoacrylate therapy for the management of gastric varices. The study was conducted in tertiary referral centers, and thirty consecutive patients with localized gastric varices received either one of the therapies, with a follow-up of 6 months after the treatment. The gastric varix obliteration rate was 94.7% in the cyanoacrylate group versus 90.9% in the coil treated patients; adverse events occurred in 12 of 30 patients, in which 11 were in the cyanoacrylate group. However, only three were symptomatic, and an additional nine had glue embolism on a CT scan, but were asymptomatic. No further adverse events occurred during follow-up (39).
Another aspect of this therapy is the combined cyanoacrylate injection and coiling, in which the synthetic fibers may function as scaffold to retain cyanoacrylate within the varix and decrease the amount of injection needed to achieve obliteration (38). Binmoeller et al. described a study that evaluated the feasibility, safety, and outcomes of transesophageal EUS-guided therapy of gastric fundal varices with combined coil and cyanoacrylate injection. There were thirty patients with hemorrhage from large gastric varices, and EUS-guided transesophageal treatment was successful in all of them. Among 24 patients with a mean follow-up of 193 days, gastric fundal varices were obliterated after a single treatment session in 23 (96%). Rebleeding occurred in four patients (16.6%), with none attributed to gastric varices. There were no procedure-related complications and no symptoms or signs of cyanoacrylate embolization (38).
In summary, there is evidence that EUS hemostasis of gastric varices is feasible and represents a potential therapy in patients with gastric variceal bleeding. However, further studies are needed to determine whether this novel approach has better outcomes over the standard endoscopic therapy with cyanoacrylate injection.
2.3 Rectal variceal bleeding
Rectal varices are usually caused by portal hypertension and may represent a potential bleeding source in cirrhotic patients. The most described endoscopic therapies for rectal variceal bleeding are band ligation and injection of sclerosant agents or cyanoacrylate (47-49). Similar to gastric varix EUS hemostasis, the EUS-guided approach in rectal variceal bleeding could deliver the precise treatment into the vessel and confirm the obliteration with Doppler analysis.
Weilert et al. first reported a case of EUS-guided coil and cyanoacrylate for a bleeding rectal varix. It described a 60-year-old woman on the waiting-list for liver transplantation who presented with hematochezia. Once the bleeding source was identified as the rectal varix, the patient underwent rectal EUS, which revealed a large rectal varix with stigmata of recent hemorrhage. Four EUS-guided 19-gauge punctures were performed alongside the varix, with the deployment of both coils and 1 mL of cyanoacrylate. Varix obliteration was confirmed with Doppler analysis and there was no bleeding during or after the procedure. The patient had no recurrent bleeding in the 12-month follow-up period (50).
Although restricted to one case study, EUS hemostasis of rectal variceal bleeding proved to be feasible and effective. Further reports and studies are needed to determine whether this novel approach will have an impact on endoscopic therapy recommendation.
3. Eus hemotasis of pseudoaneurysms
Despite being a rare and generally serious complication of pancreatitis or abdominal surgery, visceral pseudoaneurysms, when ruptured, have high mortality rates. Surgery and interventional radiology are the current treatment modalities and also have considerable morbidity and mortality. Percutaneous endovascular procedures are the modalities of choice in these lesions (51,52). EUS analysis provides great real-time visualization and proximity to the gastrointestinal pseudoaneurysms, making EUS hemostasis an alternative therapy in selected patients.
Since its first description as an aneurysmal occlusion agent, the therapeutic potential of thrombin has been increasingly recognized. Percutaneous injection of thrombin has been reported as effective in the management of femoral pseudoaneurysms (53,54). Roach et al. first described EUS hemostasis with use of thrombin injection in a 32-year-old man with a superior mesenteric artery pseudoaneurysm with recurrent bleeding episodes. Percutaneous and US/CT-guided injection was not possible due to unsuccessful selective catheterization and anatomic positioning. EUS-guided injection of 500IU of thrombin into the pseudoaneurysm was performed and with immediate obliteration observed by doppler. The patient was followed-up with for 42 weeks, and no additional treatment was required (55). Other case reports of EUS hemostasis of splenic artery pseudoaneurysms have been described with EUS-guided injection of thrombin and thrombin-collagen compound (56,57). These two reports also demonstrate immediate occlusion of the pseudoaneuryms in imaging follow-ups.
At Hospital das Clinicas, we reported a case of EUS hemostasis of a pancreatic pseudoaneurysm of the splenic artery, using EUS-guided thrombin injection (Figure 1). The case consisted of a 29-year-old man with a history of multiple episodes of acute pancreatitis, heavy alcohol consumption, and heavy smoking for 15 years. He complained of abdominal pain that worsened after meals. EUS analysis of the pancreas identified a pseudoaneurysm at the level of the pancreatic body, which was represented by a well-defined, 4.5cm maximal diameter echogenic lesion containing an anechoic area of 2 cm with flow detected by Doppler that communicated with the splenic artery by a 4.5-mm neck. An EUS-guided injection of 500IU thrombin was performed using a 22-gauge needle. Pseudoaneurysm obliteration occurred instantly, followed by a small local splenic infarction which was observed on a CT scan one week later. After a follow-up four months later, CT angiography and EUS confirmed the persistence of the occlusion (58). One important point to be considered is the presence of a small neck making the communication of the pseudoaneurysm with the artery. This increases the success of the treatment and decreases the risk of distant embolization.
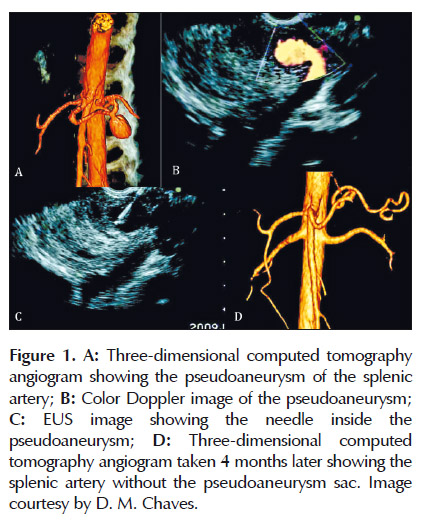
The presence of a big communication of the pseudoaneurysm with the artery may confer a high risk of complications and treatment failure. The employment of coil could be another interesting option for pseudoaneurysm with this shape.
EUS hemostasis of pseudoaneurysm with alcohol solution was described by Levy et al., in which a large pseudoaneurysm of the superior mesenteric artery was injected with 7 mL of 99% alcohol. This pseudoaneurysm had been previously treated with coiling and injection therapy during angiography with no success. After the procedure, cessation of blood flow was confirmed by EUS Doppler analysis. No complications or rebleeding occurred during 16-month follow-up (17).
There are two reports of EUS-guided injection of cyanoacrylate for the treatment of pseudoaneurysms (59,60). In one life-threatening case, Gonzalez et al. performed EUS-guided injection of cyanoacrylate into the splenic artery. The patient had chronic pancreatitis and pseudocyst. However, during the EUS drainage attempt, an intracystic pseudoaneurysm of the splenic artery was injured. A massive intracystic hemorrhage occurred and the EUS-guided puncture and cyanoacrylate injection were performed immediately. The distal portion of the splenic artery was embolized and the bleeding was controlled (59). Roberts et al. also reported a successful EUS hemostasis of a visceral pseudoaneurysm by injecting a mixture of cyanoacrylate and lipiodol directly into the lesion (60).
Literature about EUS hemostasis of pseudoaneurysms is restricted to case reports. The publications demonstrate feasibility and technical EUS advantages of such therapies. These procedures have theoretical benefits over the traditional methods and should be further explored as alternative treatment options in selected cases that are refractory to angiographic percutaneous therapy.
Final considerations
Therapeutic EUS (T-EUS) can improve the efficacy of conventional endoscopy therapy. In this review, we analyze a new application of EUS: hemostasis for GI bleeding. Regarding to the rationale justification of the EUS , we can propose the follow topics:
-
T-EUS allows for analysis of deep vascular anatomy in the intestinal wall and its correlations with minor and major vessels with closer proximity to the probe view;
-
New accessories applied to T-EUS make the technique safer and more effective;
-
Development of special glues and hemostatic agents (including mechanical agents) make T-EUS more technically feasible;
-
Interventional EUS now has an added potential therapeutic option with various T-EUS hemostasis techniques;
Finally, EUS hemostasis is already inserted among the many options of the T-EUS techniques, with the advantage of being a minimally invasive process with an acceptable safety profile. Certainly, well designed, prospective studies are urgently needed to better elucidate the efficacy and safety of T-EUS for hemostasis in cases of GI bleeding.
Conflict of Interests:
None of the authors have any conflict of interests to disclose.
REFERENCES
1. Barthet M. [Therapeutic EUS for the management of pancreatic and biliary diseases ]. Gastroenterol Clin Biol. 2009 Apr;33(4):258-65. doi: 10.1016/j.gcb .2009.02.005. [Article in French]
2. Colombato L. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension . J Clin Gastroenterol. 2007 Nov-Dec;41 Suppl 3:S344-51. [ Links ]
3. Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005 Feb;41(2):386-400. [ Links ]
4. Laine L. Endoscopic therapy for bleeding ulcers: room for improvement? Gastrointest Endosc. 2003 Apr;57(4):557-60. [ Links ]
5. Lin HJ, Hsieh YH, Tseng GY, Perng CL, Chang FY, Lee SD. A pr ospective, randomized trial of large-versus small volume endoscopic injection of epinephrine for peptic ulcer bleed ing. Am J Gastroenterol. 2002 Sep;97(9):2250-4. [ Links ]
6. Song SY, Chung JB, Moon YM, Kang JK, Park IS. Comparison of the hemostatic effect of endoscopic injection with fibrin glue and hypertonic saline-epinephrine for peptic ulcer bleeding: a prospective randomized trial. Endoscopy. 1997 Nov;29(9):827-33. [ Links ]
7. Kubba AK, Murphy W, Palmer KR. Endoscopic injection for bleeding peptic ulcer: a comparison of adrenaline alone with adrenaline plus human thrombin. Gastroenterology. 1996 Sep;111(3):623-8. [ Links ]
8. Kanai M, Hamada A, Endo Y, Takeda Y, Yamakawa M, Nishikawa H, et al . Efficacy of argon plasma coagulation in nonvariceal upper gastrointestinal bleeding. Endoscopy. 2004 Dec;36(12):1085-8. [ Links ]
9. Chau CH, Siu WT, Law BK, Tang CN, Kwok SY, Luk YW, et al. Randomized controlled trial comparing epinephrine injection plus heat probe coagulation versus epinephrine injection plus argon plasma coagulation for bleeding peptic ulcers . Gastrointest Endosc. 2003 Apr;57(4):455-61. [ Links ]
10. Jensen DM, Kovacs TO, Jutabha R, Machicado GA, Gralnek IM, Savides TJ, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology. 2002 Aug;123(2):407-13. [ Links ]
11. Laine L, Estrada R. Randomized trial of normal saline solution injection versus bipolar electrocoagulation for treatment of patients with high-risk bleeding ulcers: is local tamponade enough? Gastrointest Endosc. 2002 Jan;55(1):6-10. [ Links ]
12. Chou YC, Hsu PI, Lai KH, Lo CC, Chan HH, Lin CP, et al. A prospective, randomized trial of endoscopic hemoclip placement and distilled water injection for treattment of high-risk bleeding ulcers. Gastrointest Endosc. 2003 Mar;57(3):324-8. [ Links ]
13. van Leerdam ME, Vreeburg EM, Rauws EA, Geraedts AA, Tijssen JG, Reitsma JB, et al. Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003 Jul;98(7):1494-9. [ Links ]
14. Targownik LE, Nabalamba A. Trends in management and outcomes of acute nonvariceal upper gastrointestinal bleeding: 1993–2003. Clin Gastroenterol Hepatol. 2006 Dec;4(12):1459-1466. [ Links ]
15. Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010 Jan 19;152(2):101-13. doi: 10.7326/0003 4819-152-2-201001190-00009. [ Links ]
16. Elmunzer BJ, Pollack MJ, Trunzo JA, Schomisch SJ, Wong RC, Faulx AL, et al. Initial evaluation of a novel, prototype, forward-viewing echoendoscope in a porcine arterial bleeding model (with video). Gastrointest Endosc. 2010 Sep;72(3):611-4. doi: 10.1016/j. gie .2010.04.053. [ Links ]
17. Levy MJ, Wong Kee Song LM, Farnell MB, Misra S, Sarr MG, Gostout CJ. Endoscopic ultrasound (EUS)-guided angiotherapy of refractory gastrointestinal bleeding. Am J Gastroenterol. 2008 Feb;103(2):352-9. [ Links ]
18. Gonzalez JM, Giacino C, Pioche M, Vanbiervliet G, Brardjanian S, Ah-Soune P, et al. Endoscopic ultrasound-guided vascular therapy: is it safe and effective? Endoscopy. 2012 May;44(5):539-42. doi: 10.1055/s-0031-1291609. [ Links ]
19. Fockens P, Meenan J, van Dullemen HM, Bolwerk CJ, Tytgat GN. Dieulafoys disease: endosonographic detection and endosonography- guided treatment. Gastrointest Endosc. 1996 Oct;44(4):437-42. [ Links ]
20. Ribeiro A, Vazquez-Sequeiros E, Wiersema MJ. Doppler EUS guided treatment of gastric Dieulafoys lesion. Gastrointest Endosc. 2001 Jun;53(7):807-9. [ Links ]
21. De Franchis R, Primignani M. Endoscopic treatments for portal hypertension. Semin Liver Dis. 1999;19(4):439-55. [ Links ]
22. Helmy A, Hayes PC. Review article: current endoscopic therapeutic options in the management of variceal bleeding. Aliment Pharmacol Ther. 2001 May;15(5):575-94. [ Links ]
23. Marrero JA, Scheiman JM. Prevention of recurrent variceal bleeding: as easy as A.P.C.? Gastrointest Endosc. 2002 Oct;56(4):600-3. [ Links ]
24. Sarin SK, Govil A, Jain AK, Guptan RC, Issar SK, Jain M, et al. Prospective randomized trial of endoscopic sclerotherapy versus variceal band ligation for esophageal varices: influence on gastropathy, gastric varices and variceal recurrence. J Hepatol. 1997 Apr;26(4):826-32. [ Links ]
25. Hou MC, Lin HC, Lee FY, Chang FY, Lee SD. Recurrence of esophageal varices following endoscopic treatment and its impact on rebleeding: comparison of sclerotherapy and ligation. J Hepatol. 2000 Feb;32(2):202-8. [ Links ]
26. Irisawa A, Obara K, Bhutani MS, Saito A, Shishido H, Shibukawa G, et al. Role of para-esophageal collateral veins in patients with portal hypertension based on the results of endoscopic ultrasonography and liver scintigraphy analysis. J Gastroenterol Hepatol. 2003 Mar;18(3):309-14. [ Links ]
27. Irisawa A, Saito A, Obara K, Shibukawa G, Takagi T, Shishido H, et al. Endoscopic recurrence of esophageal varices is associated with the specific EUS abnormalities: severe peri-esophageal collateral veins and large perforating veins. Gastrointest Endosc. 2001 Jan;53(1):77-84. [ Links ]
28. Krige JE1, Bornman PC, Goldberg PA, Terblanche J. Variceal rebleeding and recurrence after endoscopic injection sclerotherapy: a prospective evalua tion in 204 patients. Arch Surg. 2000 Nov;135(11):1315-22.
29. Lahoti S, Catalano MF, Alcocer E, Hogan WJ, Geenen JE. Obliteration of esophageal varices using EUS-guided sclerotherapy with color Doppler. Gastrointest Endosc. 2000 Mar;51(3):331-3. [ Links ]
30. de Paulo GA1, Ardengh JC, Nakao FS, Ferrari AP. Treatment of esophage al varices: a randomized controlled trial comparing endoscopic sclerotherapy and EUS-guided sclerotherapy of esophageal collateral veins. Gastrointest Endosc. 2006 Mar;63(3):396-402; quiz 463.
31. Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992 Dec;16(6):1343-9. [ Links ]
32. Trudeau W, Prindiville T. Endoscopic injection sclerosis in bleeding gastric varices. Gastrointest Endosc. 1986 Aug;32(4):264-8. [ Links ]
33. Soehendra N, Nam VC, Grimm H, Kempeneers I. Endoscopic obliteration of large esophagogastric varices with bucrylate. Endoscopy. 1986 Jan;18(1):25-6. [ Links ]
34. De Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005 Jul;43(1):167-76. [ Links ]
35. Levy MJ, Wong Kee Song LM. EUS-guided angiotherapy for gastric varices: coil, glue, and sticky issues. Gastrointest Endosc. 2013 Nov;78(5):722-5. doi: 10.1016/j.gie .2013.07.004. [ Links ]
36. Seewald S, Ang TL, Imazu H, Naga M, Omar S, Groth S, et al. A standardized injection technique and regimen ensures success and safety of N-butyl-2-cyanoacrylate injection for the treatment of gastric fundal varices (with videos). Gastrointest Endosc. 2008 Sep;68(3):447-54. doi: 10.1016/j. gie .2008.02.050. [ Links ]
37. Irani S, Kowdley K, Kozarek R. Gastric varices: an updated review of management . J Clin Gastroenterol. 2011 Feb;45(2):133-48. doi: 10.1097/MCG .0b013e3181fbe249. [ Links ]
38. Binmoeller KF1, Weilert F, Shah JN, Kim J. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos). Gastrointest Endosc. 2011 Nov;74(5):1019-25. doi: 10.1016/j. gie .2011.06.030.
39. Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, Subtil-Inigo JC, Junquera-Florez F, Gornals JB, et al. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: a multicenter study (with videos) . Gastrointest Endosc. 2013 Nov;78(5):711-21. doi: 10.1016/j.gie .2013.05.009. [ Links ]
40. Lee YT, Chan FK, Ng EK, Leung VK, Law KB, Yung MY, et al. EUS guided injection of cyanoacrylate for bleeding gastric varice s. Gastrointest Endosc. 2000 Aug;52(2):168-74. [ Links ]
41. Romero-Castro R, Pellicer-Bautista FJ, Jimenez-Saenz M, Marcos-Sanchez F, Caunedo-Alvarez A, Ortiz-Moyano C, et al. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: results in 5 cases. Gastrointest Endosc. 2007 Aug;66(2):402-7. [ Links ]
42. Levy MJ, Wong Kee Song LM, Kendrick ML, Misra S, Gostout CJ. EUS guided coil embolization for refractory ectopic variceal bleeding (with videos). Gastrointest Endosc. 2008 Mar;67(3):572-4. [ Links ]
43. Romero-Castro R, Pellicer-Bautista F, Giovannini M, Marcos-Sánchez F, Caparros-Escudero C, Jiménez-Sáenz M, et al. Endoscopic ultrasound (EUS)-guided coil embolization therapy in gastric varic es. Endoscopy. 2010;42 Suppl 2:E35-6. doi: 10.1055/s-0029-1215261. [ Links ]
44. Lunderquist A, Börjesson B, Owman T, Bengmark S. Isobutyl 2-cyanoacrylate (bucrylate) in obliteration of gastric coronary vein and esophageal varic es. AJR Am J Roentgenol. 1978 Jan;130(1):1-6. [ Links ]
45. Witt WS, Goncharenko V, OLeary JP, Muhletaler C, Gerlock AJ Jr. I nterruption o f gastroesophageal varices: seal coil technique. AJR Am J Roentgenol. 1980 Oct;135(4):829-33. [ Links ]














