Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Gastroenterología del Perú
versión impresa ISSN 1022-5129
Rev. gastroenterol. Perú vol.34 no.4 Lima oct. 2014
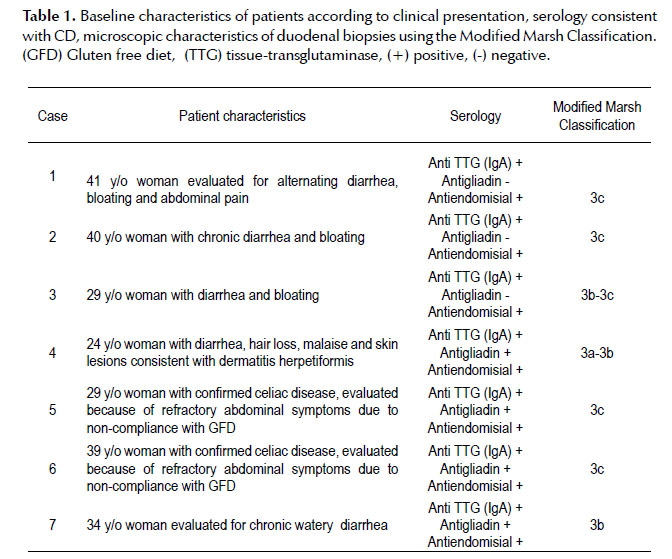
Leopard skin sign: the use of narrow-band imaging with magnification endoscopy in celiac disease
Signo de la piel de leopardo: el uso de endoscopía con magnificación y narrow banding en enfermedad celiaca
Asadur J. Tchekmedyian 1, Emmanuel Coronel 2, Frank Czul 3
1 Universidad de la República, Depar tamento de Gastroenterología Prof. Henry Cohen, Hospital de Clínicas. Montevideo, Uruguay.
2 Division of Gastroenterology, Hepatology and Nutrition, Depar tment of Medicine, University of Chicago. Chicago, EE UU.
3 Miller School of Medicine, Division of Gastroenterology, Depar tment of Medicine, University of Miami. Miami, EE UU.
ABSTRACT
Celiac Disease (CD) is an immune reaction to gluten containing foods such as rye, wheat and barley. This condition affects individuals with a genetic predisposition; it targets the small bowel and may cause symptoms including diarrhea, malabsorption, weight loss, abdominal pain and bloating. The diagnosis is made by serologic testing of celiac-specific antibodies and confirmed by histology. Certain endoscopic characteristics, such as scalloping, reduction in the number of folds, mosaic-pattern mucosa or nodular mucosa, are suggestive of CD and can be visualized under white light endoscopy. Due to its low sensitivity, endoscopy alone is not recommended to diagnose CD; however, enhanced visual identification of suspected mucosal abnormalities through the use of new technologies, such as narrow band imaging with magnification (NBI-ME), could assist in targeting biopsies and thereby increasing the sensitivity of endoscopy. This is a case series of seven patients with serologic and histologic diagnoses of CD who underwent upper endoscopies with NBI-ME imaging technology as part of their CD evaluation. By employing this imaging technology, we could identify patchy atrophy sites in a mosaic pattern, with flattened villi and alteration of the central capillaries of the duodenal mucosa. We refer to this epithelial pattern as “Leopard Skin Sign”. Since epithelial lesions are easily seen using NBI-ME, we found it beneficial for identifying and targeting biopsy sites. Larger prospective studies are warranted to confirm our findings.
Key words: Celiac disease; Endoscopy; Duodenum; Narrow band imaging; Diagnosis (source: MeSH NLM).
ABSTRACT
La enfermedad celiaca (EC) es una reacción inmune a los alimentos que contienen gluten como el centeno, el trigo y la cebada. Esta condición afecta a las personas con predisposición genética, comprometiendo al intestino delgado causando síntomas como diarrea, mala absorción, pérdida de peso, dolor abdominal y meteorismo. El diagnóstico se hace con estudios serológicos de anticuerpos específicos celiacos y es confirmado por histología. Algunas características endoscópicas tales como “scalloping”, reducción en el número de pliegues, patrón mucoso tipo mosaico o mucosa nodular, son sugestivos de EC y se pueden observar con endoscopía de luz blanca. Debido a su baja sensibilidad la endoscopía por sí sola no se recomienda para diagnosticar EC, sin embargo, una visualización cuidadosa de las anormalidades mucosas sospechosas a través de nuevas tecnologías como “Narrow Band Imagining” con magnificación (NBI-ME) puede ayudar a dirigir las biopsias y así incrementar la sensibilidad de la endoscopía. Esta es una serie de siete casos con diagnóstico serológico e histológico de EC a quienes se les realizó una endoscopía digestiva alta con NBI-ME. En ellos se pudo identificar sitios de atrofia parcelar en un patrón de mosaico, con vellosidades aplanadas y alteración de los capilares de la mucosa duodenal. Nos referimos a esta alteración como el “Signo de la Piel de Leopardo”. Como las lesiones epiteliales se ven fácilmente usando NBI-ME, lo encontramos beneficioso para identificar y dirigir los sitios donde tomar las biopsias. Estudios prospectivos más grandes deben realizarse para confirmar nuestros hallazgos.
Palabras clave: Enfermedad celíaca; Endoscopía; Duodeno; Imagen de banda estrecha; Diagnóstico (fuente: DeCS BIREME).
INTRODUCTION
Celiac disease (CD) is defined as an immune reaction to gluten containing foods such as rye, wheat and barley. This condition affects individuals with a genetic predisposition; it targets the small bowel and may cause symptoms including diarrhea, malabsorption, weight loss, abdominal pain and bloating. A CD diagnosis is made by serologic testing of celiac-specific antibodies in individuals following a gluten containing diet and is confirmed by endoscopy with multiple duodenal biopsies for histology. The mainstay of therapy is a gluten- free diet (GFD) which requires significant education and commitment (1,2).
The role of endoscopy in CD has been evaluated in multiple studies, mainly to describe the pattern of mucosal injury and test the accuracy of visualization of villous atrophy. In CD, the intestinal damage can have a patchy distribution, and the macroscopic features can be related to the degree of severity of the histological lesions. Several endoscopic features reflect the presence Narrow band imaging in celiac disease of villous atrophy; however, their sensitivity varies from
50% to 94%. Consequently, endoscopy alone is not recommended to establish a CD diagnosis. Endoscopy plays an important role, though, in the diagnosis and management of this disease (1,3,4). Enhanced visual identification of suspected mucosal atrophy could assist in targeting biopsies and increase the sensitivity of endoscopy, reducing the need of random biopsies (5).
Narrow band imaging (NBI) is a technology that uses two different light filters reducing the light wavelength to 415 and 540 nanometers; the penetration of the shorter wavelength blue light is limited to the mucosa. This corresponds to the main and secondary hemoglobin absorption improving the contrast between the sub mucosal vasculature and mucosa, enhancing the mucosal structure (6).
This is a case series of seven patients with a serologic and histologic diagnosis of CD who underwent evaluation with narrow-band imaging with magnification endoscopy (NBI-ME) prior to duodenal biopsy taking.
CASE SERIES
This case series was obtained from the Department of Gastroenterology of the “Hospital de Clinicas” in Montevideo, Uruguay. All patients had serology results consistent with CD. Two patients had established CD diagnoses, positive serologies and biopsies, and were evaluated due to refractory symptoms. The five remaining patients were initially diagnosed with CD by serology and confirmed with endoscopy and histology. Patients underwent upper endoscopies with NBI prior to duodenal biopsies as part of their evaluation. Table 1 represents patient characteristics and histologic findings using the Modified Marsh Classification (7).
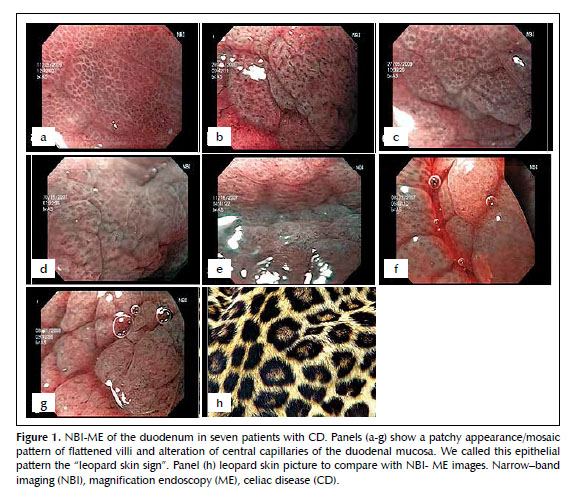
We used a magnification gastroscope (GIF Q160Z, Olympus, Tokyo, Japan) with optical magnification of 115x and NBI technology (Figure 1). At the time of endoscopic evaluation with white light, it was difficult to find all the characteristic endoscopic changes suggestive of CD such as: scalloping and reduction in the number of folds, mosaic-pattern mucosa, and nodular mucosa. We used NBI-ME to target the biopsy sites and obtained multiple biopsies (at least 4). All seven patients had severe intestinal atrophy by histology, and through NBI-ME, all displayed patchy atrophy sites in a mosaic pattern, with flattened villi and alteration of central capillaries of the duodenal mucosa. We called this epithelial pattern the “Leopard Skin Sign” (Figures 1 and 2). We found NBI useful for identifying and targeting biopsy sites since the pattern of epithelial lesions is easily observed using this technology.
DISCUSSION
It is controversial whether CD can be diagnosed without a duodenal biopsy. The European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) proposed that it may be possible to avoid any intestinal biopsy in children who meet the following criteria: characteristic symptoms of CD, Tissue Transglutaminase (TTG) IgA levels > 10 × upper limit of normal (confirmed with a positive anti-endomysial antibody in a different blood sample), and positive HLA-DQ2 (triple testing). There is no sufficient data to recommend triple testing as a diagnostic strategy since there is no standardization of TTG assays and there is not enough data in unselected populations such as family members of individuals with confirmed CD (1).
Endoscopic evaluation with histopathological sampling is an important step for patients undergoing evaluation for CD or as part of their management. Routinely, white light endoscopy is used to study patients with CD, but it has been found to have a low sensitivity in detecting typical mucosal changes (4). New imaging technologies have been used to facilitate the diagnosis of different digestive conditions such as early dysplasia of the intestinal tract (6,8). There is not sufficient data, though, to recommend its systematic use for diagnosis/ confirmation of CD. Similar to a prior study that found magnification endoscopy improved the sensitivity and specificity of endoscopy in the diagnosis of CD (9), we found that combining NBI with this technology useful for identifying and targeting biopsy sites as well as enhancing areas of mucosal atrophy for better visualization.
Better assessment of mucosal healing in patients with CD is warranted. Recent findings described by Lebwohl et al., describe an increased risk of lymphoproliferative malignancy in patients with CD and persistent villous atrophy, hence the importance of using enhanced imaging technologies to assess mucosal architecture (10).
The small number of patients is a limitation of our case series; larger prospective studies are necessary to confirm our present findings.
Acknowledgements:
To: Prof. Dr. Henry Cohen, Dr. Julio Chilewski, Dr. Juan Pablo Gutierrez, Dr. Andres Taullard and Dr. Virgina Lopez (Hospital de Clinicas, Montevideo, Uruguay) who have helped by obtaining data for the preparation of this manuscript.
Conflict of interest:
None of the authors have any conflicts of interest to disclose.
REFERENCES
1. Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG Clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013 May;108(5):656-76; quiz 677. doi: 10.1038/ajg.2013.79.
2. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012 Dec 20;367(25):2419-26. doi: 10.1056/ NEJMcp1113994.3. Harris LA, Park JY, Voltaggio L, Lam-Himlin D. Celiac disease: clinical, endoscopic, and histopathologic review. Gastrointest Endosc. 2012 Sep;76(3):625-40. doi: 10.1016/j. gie.2012.04.473.
4. Lecleire S, Di Fiore F, Antonietti M, Savoye G, Lemoine F, Le Pessot F, et al. Endoscopic markers of villous atrophy are not useful for the detection of celiac disease in patients with dyspeptic symptoms. Endoscopy. 2006 Jul;38(7):696-701.
5. Lo A, Guelrud M, Essenfeld H, Bonis P. Classification of villous atrophy with enhanced magnification endoscopy in patients with celiac disease and tropical sprue. Gastrointest Endosc. 2007 Aug;66(2):377-82.
6. Subramanian V, Ragunath K. Advanced endoscopic imaging: a review of commercially available technologies. Clin Gastroenterol Hepatol. 2014 Mar;12(3):368-76.e1. doi: 10.1016/j.cgh.2013.06.015.
7. Oberhuber G, Caspary WF, Kirchner T, Borchard F, Stolte M; German Society for Pathology Task Force on Gastroenterologic Pathology. [Diagnosis of celiac disease and sprue. Recommendations of the German Society for Pathology Task Force on Gastroenterologic Pathology]. Pathologe. 2001 Jan;22(1):72-81. [Article in German]
8. Li HY, Dai J, Xue HB, Zhao YJ, Chen XY, Gao YJ, et al. Application of magnifying endoscopy with narrow-band imaging in diagnosing gastric lesions: a prospective study. Gastrointest Endosc. 2012 Dec;76(6):1124-32. doi:10.1016/j.gie.2012.08.015.9. Banerjee R, Shekharan A, Ramji C, Puli SR, Kalapala R, Ramachandani M, et al. Role of magnification endoscopy in the diagnosis and evaluation of suspected celiac disease: correlation with histology. Indian J Gastroenterol. 2007 Mar- Apr;26(2):67-9.
10. Lebwohl B, Granath F, Ekbom A, Smedby KE, Murray JA, Neugut AI, et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med. 2013 Aug 6;159(3):169-75. doi:10.7326/0003-4819-159-3-201308060-00006.
Correspondence:
Dr. Asadur Jorge Tchekmedyian
Rambla República del Perú 1413 – Ap. 201. Montevideo 11300. Uruguay
E-mail: asadur@adinet.com.uy
Recibido: 05-08-2014
Aprobado: 06-10-2014