Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Gastroenterología del Perú
Print version ISSN 1022-5129
Rev. gastroenterol. Perú vol.36 no.1 Lima Jan./Mar. 2016
ARTÍCULOS ORIGINALES
Colorectal cancer in the young: clinicopathologic features and prognostic factors from a cancer institute in Peru
Cáncer colorrectal en los jóvenes: factores pronósticos y características clínico patológicas en un instituto del cáncer de Perú
Rossana Ruiz1, Luis Taxa2, Eloy F. Ruiz3, Raúl Mantilla4, Luis Casanova1, Paola Montenegro1
1. Departamento de Oncología Médica. Instituto Nacional de Enfermedades Neoplásicas, Lima, Perú.
2. Departamento de Patología. Instituto Nacional de Enfermedades Neoplásicas, Lima, Perú.
3. Facultad de Medicina. Universidad Peruana Cayetano Heredia, Lima, Perú.
4. Dirección de Educación, Instituto Nacional de Enfermedades Neoplásicas. Lima, Perú.
ABSTRACT
Objective: To determine clinicopathological features and prognostic factors among young colorectal cancer (CRC) patients in a Peruvian Cancer Institute. Methods: Data of patients 40 years or younger, admitted between January 2005 and December 2010, were analyzed. Results: During the study period, 196 young patients with CRC were admitted. The tumor was located in the rectum, left colon and right colon in 45.9%, 28.6% and 25.5% of cases. Family history of CRC was found in 13.2% and an autosomal pattern of inheritance, in 8.6% of the cases. The most common symptoms were pain (67.9%) and bleeding (67.3%). The majority (63.1%) of colon cancer cases and more than a third (34.4%) of rectal cancer cases were diagnosed in stage III or IV. The histologic type was tubular, mucinous and signet ring cell adenocarcinoma in 73.5%, 14.8% and 8.6%, respectively. The depth of invasion was T3 in 21.4% and T4 in 53%. Nodal involvement was detected in 44.5%. Five-year overall survival (OS) was 44.3%. In the multivariate analysis, only the stage resulted an independent prognostic factor for survival. Conclusions: CRC in Peruvian young patients is mostly sporadic. It presents more often in the distal colon or rectum and at advanced stages of the disease. Mucinous and signet ring cell carcinoma were frequent histological types. Five-year OS stage by stage is similar to that reported in the literature for older patients. Stage was the only independent prognostic factor for survival.
Key words: Colorectal neoplasms; Survival; Prognosis; Young adult; Peru (source: MeSH NLM).
RESUMEN
Objetivo: Determinar las características clínicopatológicas y factores relacionados con el pronóstico del cáncer colorrectal (CCR) en los pacientes jóvenes del Instituto Nacional de Enfermedades Neoplásicas (INEN). Material y métodos: Se analizaron retrospectivamente los datos de los pacientes de 40 o menos años admitidos entre enero del 2005 y diciembre del 2010. Resultados: Se incluyeron 196 pacientes. La localización correspondió al recto, colon izquierdo y colon derecho en 45,9%, 28,6% y 25,5%. En 13,2% hubo antecedentes familiares de CCR y en 8.6%, un patrón de herencia autosómico dominante. Los síntomas más frecuentes fueron dolor (67,9%) y sangrado (67,3%). El 63,1% de los casos de cáncer de colon y el 34,4% de los casos de cáncer de recto, se diagnosticaron en estadio clínico (EC) III o IV. El tipo histológico correspondió a adenocarcinoma tubular, mucinoso y de células en anillo de sello en 73,5%, 14,8% y 8,6%, respectivamente. La profundidad de la invasión fue de T3 en 21,4% y de T4 en 53%. En 44,5% de los casos hubo compromiso ganglionar. La sobrevida global (SG) a 5 años fue de 44,3%. En el análisis multivariado, el estadio resultó ser un factor pronóstico independiente. Conclusiones: El CCR en jóvenes es en su mayoría esporádico, se presenta con mayor frecuencia en el colon distal o recto y en estadios avanzados. El carcinoma mucinoso y de células en anillo de sello fueron tipos histológicos frecuentes. La SG comparada por estadios es similar a la reportada en la literatura. El EC fue el único factor pronóstico independiente para sobrevida.
Palabras clave: Neoplasias colorrectales; Supervivencia; Pronóstico; Adulto joven; Peru (fuente: DeCS BIREME).
INTRODUCTION
Worldwide, colorectal cancer (CRC) holds the third place among the most commonly diagnosed malignancies with an incidence of 17.2 new cases per 100,000 (1). In Peru, it ranked fourth in 2012, with 3,000 new cases and 1,800 deaths (1). In Lima, about 900 cases per year are registered. Patients treated at Instituto Nacional de Enfermedades Neoplásicas (INEN) account for more than a half of these (2).
Typically, CRC affects adults in their sixth decade of life, with over 90% of cases diagnosed in those older than 55 years old (3). Thus, in the absence of risk factors, screening is recommended from 50 years old onwards (4). Nevertheless, epidemiological studies suggest that CRC incidence among the young is increasing (5-8). Most of the series consider 40 years as the cut-off limit of age to define a patient as a young one (9-11). According to the literature, the percentage of young patients with CRC varies between 0.4% and 35.6%, with a mean of 7% (10). In our country, a retrospective study showed that 96.8% of CRC cases were diagnosed on patients over 40 years old (12).
According to the literature, most cases are sporadic (10), as family history of CRC has been found only in 20 to 30% (13). Eighty-five percent of patients are symptomatic (14), with abdominal pain and bleeding as the most common symptoms (10). The rectum and left colon constitute the most frequent locations (10). The prognosis of CRC in the young is still controversial. Several studies agree that tumors in this age-group are diagnosed in later stages, with higher histological grade and more frequently of mucinous and signet ring cell types, when compared to older adults (10). All of these features, contributing to a poorer prognosis (9,10,15,16). Still, other studies report similar outcomes when compared to older patients (17-20).
Due to the disagreements within the literature and adding the fact that, so far, no study on CRC in young patients has been published in our country, the present study aims to determine the clinicopathological features and factors related to CRC prognosis in patients aged 40 or less from our institute.
METHODS
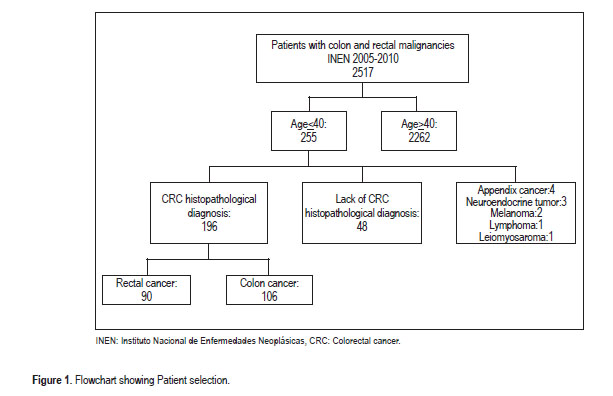
Between January 2005 and December 2010, 2,517 patients were diagnosed with colon and rectum malignancies at INEN. Two-hundred and fifty-five patients were 40 years old or younger. From this group, 48 subjects without histopathological confirmation of diagnosis at our institution were excluded, as well as other 11, for corresponding to other diagnosis (Figure 1). The 196 cases with confirmed CRC were selected and evaluated.
Clinicopathological findings were obtained from medical records. A database was developed including the following variables: age, gender, personal and family cancer history, time interval between onset of symptoms and diagnosis (TISD), symptoms, hemoglobin (Hb), carcinoembryonic antigen level (CEA), location, pathological findings, TNM stage (21) and treatment. In cases where pathological findings were not clear, an expert pathologist reexamined the samples.
Descriptive analysis was performed through frequency distribution tables. For the overall survival (OS) analysis, the follow-up period was defined as the time elapsed between the date of diagnosis and the date of death or last contact. Survival curves were estimated using the Kaplan-Meier method and differences among curves within different variables were established with the log-rank test. Prognostic factors were identified through Cox regression model. A level of p<0,05 was considered significant. The statistical package SPSS 12.0 (IBM SPSS Statistics for Windows, Version 19.0. Armonk,NY: IBM Corp) was used for the data analysis.
Universidad Peruana Cayetano Heredia Ethics Committee and the INEN Research Departmentapproved the study protocol. The confidentiality of the data obtained from the medical histories was kept.
RESULTS
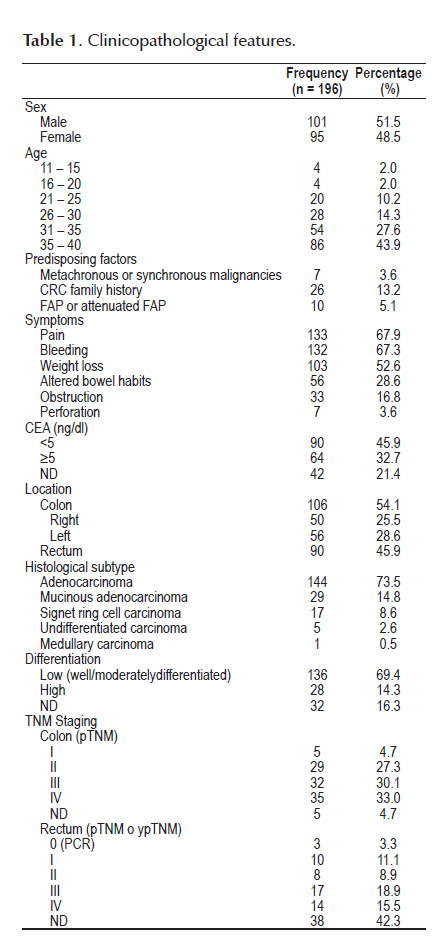
Features of the 196 selected young patients are shown on Table 1. Fifty-one percent (n=101) were men. The average age was 32.9 years (range 11-40). Family history of cancer was found in 36.22% (n=71), and 13.2% (n=26) had CRC family history. Only in 8.67% of patients (n=17), an autosomal dominant inheritance pattern was detected. Average TISD was 8.3 months (range 0.5-48 months). The most frequent signs and symptoms were pain (67.9%) and bleeding (67.3%). Bowel obstruction and/or perforation accounted for 20.4%. Forty-four percent of cases (n=87) presented with anemia. Only 32.7% (n=64) displayed an abnormally increased CEA level (≥5ng/ ml). In 106 patients (54.1%), the tumor was located in the colon (25.5% right colon and 28.6% left colon), and in 90 patients (45.9%) in the rectum. Most of colon cancer cases (63.1%) and almost a third of the rectal ones (34.4%), were diagnosed in stage III or IV.
Histology corresponded to tubular adenocarcinoma, mucinous adenocarcinoma and signet ring cell carcinoma in 73.5%, 14.8% and 8.6%, respectively. In 14.3% of the cases, lesions were of high grade.
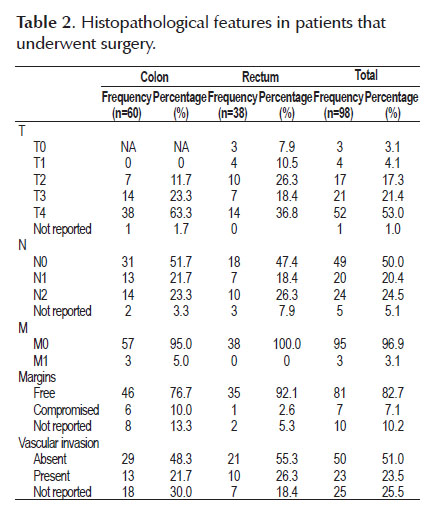
In the 98 patients that underwent a surgical procedure with curative intent, additional histopathological features were determined (Table 2). The tumor had a T4 depth of invasion in 52 of the cases (53.0%) and T3in 21 (21.4%). Forty-four cases (44.9%) were N1 or N2. The average number of resected lymph nodes was 48 (range 10-192). Vascular invasion was present in 23 cases (23.5%).
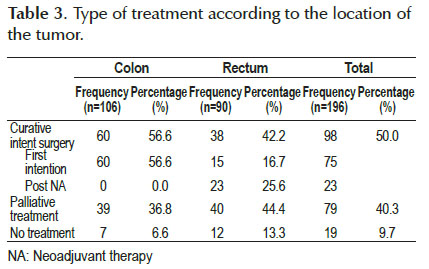
Primary treatment is shown in Table 3. Half of the patients underwent curative-intent surgery. Out of the 106 colon cancer patients, 60 (56.6%), had curative- intent surgery. From the 90 patients with rectal cancer, 38 (42.2%) had curative-intent surgery. Out of the later, 15 (39.5%) were primarily intervened and 23 (60.5%) underwent surgery after receiving neoadjuvant (NA) chemoradiation. Initially, 52 patients received chemoradiation with NA intention; within those, 17 (32.6%) presented progressive disease, 10 (19.2%) refused treatment or were lost to follow up, two patients had insufficient surgery and only 23 (44.2%) underwent curative-intent surgery and correspond to the already mentioned ones.
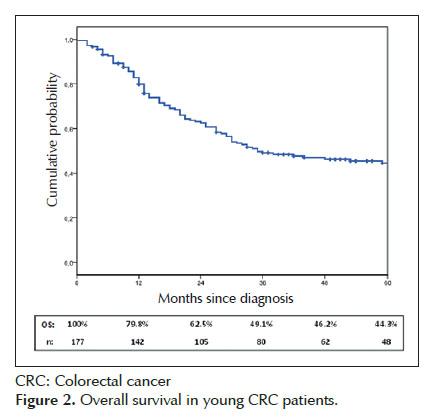
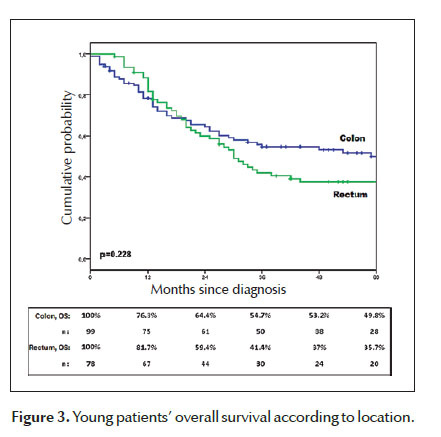
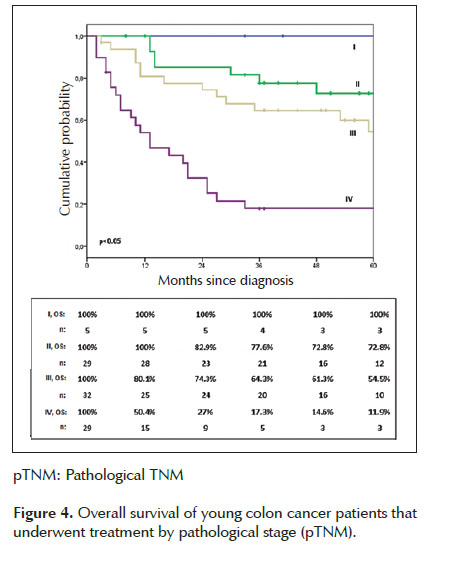
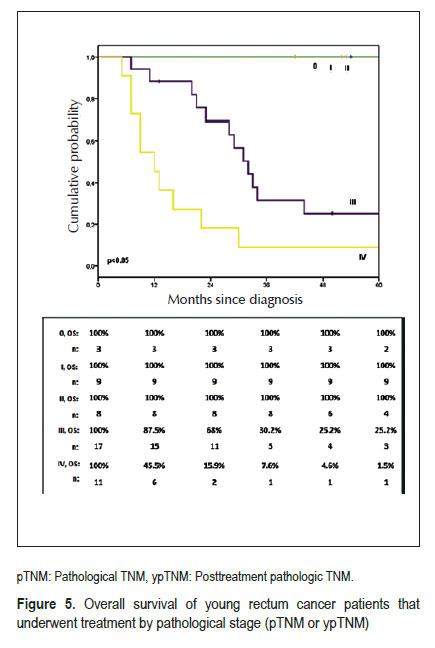
Within young CRC treated patients, 5-year OS was 44.3% (Figure 2). There was not statistic difference between colon and rectal cancer OS (Figure 3). Five- year OS for colon cancer patients that underwent surgery with curative-intent was estimated in 69.4%. According to the pathological staging, the survival was 72.8%, 54.5% and 11.9% for the stages II, III and IV respectively. There were no deaths among patients in stage I (Figure 4). A significant difference in survival was found between stages. Five-year OS for rectal cancer patients that underwent curative-intent surgery was estimated in 67.4%. Twenty-five percent and 1.5% of stage III and IV patients, respectively, were alive after 5 years. There were no deaths among Stage 0, I and II. A significant difference in survival was found between stages (Figure 5).
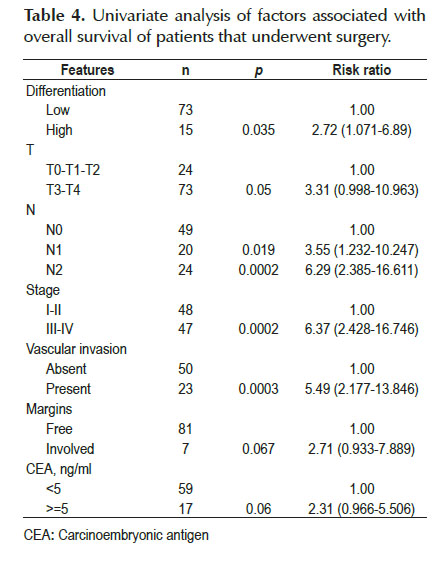
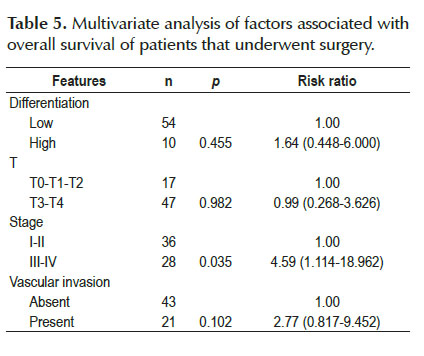
In the univariate analysis, high histological grade, N1 and N2 nodal involvement, stage III-IV, as well as the presence of vascular invasion, were factors that increased the risk of death and were therefore related to survival. No significant association was found with other factors such as depth of invasion, margin status and CEA level (Table 4). In the multivariate analysis, only the stage resulted an independent prognostic factor (HR=4.59, 95% CI 1.114-18.962) (Table 5).
DISCUSSION
The present study is the first on CRC young patients in Peru and, as far as we know, the series with the largest number of young patients in Latin America.
Patients 40 years or younger accounted for 10% of all CRC cases diagnosed during the study period. This value is situated within the range of 0.4-35.6% reported by a previous revision (10). Several reports indicate a rise in CRC incidence in young patients (5-8,16). Possible explanations for such trend include the increase of known CRC risk factors, as obesity, sedentary lifestyle and western diet. Furthermore, routines creening is limited to older patients (8).
In the general population as in young patients, most of CRC cases are sporadic (13). Twenty-five percent correspond to familial clustering, due to unknown genetic mutations without an established inheritance pattern; and 5%, to hereditary CRC predisposition syndromes, produced by germline mutations in high penetrance genes (22,23). A review of the literature found that on average, 22% of young patients had CRC family history and 16%, predisposing factors, such as familial adenomatous polyposis (FAP), Lynch syndrome or inflammatory bowel disease (10). In our series, CRC family history or any predisposing factor were detected in 13% and 6% of patients, respectively. These percentages, which are relatively low, may have resulted from the incomplete family history information.
The literature and our study suggest that a significant delay in CRC diagnosis exists among young patients (10,13,16), being 6 months the average TISD (10). In our population, on average, 8 months elapsed between initiation of symptoms and diagnosis. There are patient-related as well as physician-dependent factors, such as limited access to healthcare and a low suspicion index, which could condition this delay. In our patients, the main symptoms were pain and bleeding. Notably, 20.4% presented as an emergency, due to bowel obstruction and/or perforation. This value is increased when compared to CRC in general population (24), but similar to the one reported for young patients (16). This finding may be related to the large amount of patients that presented a T4 depth of invasion in the intestinal wall.
Various studies indicate that carcinogenesis mechanisms and, therefore, biological, clinical and epidemiological CRC features vary according to their location (25). Among those, age at diagnosis is an important factor. With aging, an increase in proximal tumors and a decrease in rectal tumors has been reported (26). In that regard, average age at diagnosis for rectal cancer is minor (63 years old in men and 65 in women) than for colon cancer (69 and 73 years old, respectively) (27). Moreover, a study that assessed almost 10,000 CRC cases in the U.S. reported a significant and progressive decrease in age as the tumors were more distal, from the cecum, ascending, transverse, descending and sigmoid colon until the rectum (28). According to these findings, the frequency of right colon, left colon and rectal cancer within our series was 25.5%, 28.6% and 45.9%, respectively. Such results are similar to other young patients series (7,14,15,20) and differ widely from those reported for the general population, where right colon cancer is more frequent, accounting for up to 42% of cases (26).
Significant differences among histopathological features have been reported. Numerous studies agree on the existence of a higher frequency of mucinous adenocarcinoma and signet ring cell carcinoma compared to older patients. Also, regarding differentiation grade, it would likely exist a higher frequency of poorly differentiated or high- grade malignancies. One of the largest series, based on data from a single Taiwanese institution, which included 5,168 patients, found that the mucinous adenocarcinoma and poorlydifferentiated neoplasms frequency was inversely proportional to age (29). Concerning young population, a review of the literature published in 2004, which included 55 articles (10), reported that on average, 21% of patients had mucinous adenocarcinomas; 3%, signet ring cell carcinomas; and 27%, high-grade carcinomas. In 2011, You et al, based in the U.S. National Cancer Database, published the study with the largest amount of young patients. It assessed over half a million CRC patients, from which, over 60,000were under 50 years old. The investigators found that 13% of the young patients and 10% of the older ones, had mucinous adenocarcinoma; likewise, 20% of the young patients and 18% of the older ones, presented high-grade neoplasms. Such differences reached major statistical significance (7). Other publications found significant differences as well, when comparing the aforementioned features between young and older patients (15,18,20). Our series reports that 15%, 9% and 14% of cases corresponded, to mucinous adenocarcinoma, signet ring cell carcinoma and high-grade neoplasms, respectively. A previous Peruvian study, showed that signet ring cell carcinomas accounted for 5.09% of CRC cases diagnosed at INEN (30). This histological subtype is significantly associated to a higher histological grade and a more advanced stage at diagnosis and, independently associated to a higher mortality risk (31). Within our series, 10 out of 17 patients were stage III or IV at diagnosis and, all of them, but one, died because of the disease. The high proportion of signet ring cell carcinomas reported by our series is remarkable. A study which included 1,522 CRC cases with signet ring cell histology throughout 10 years, found that its incidence is increasing (32). Despite that fact that this might explain our findings, a molecular analysis is mandatory in signet ring cells carcinoma cases to determine possible variations in their genetic pattern.
Vascular invasion, mostly when produced at the level of extramural veins, is recognized as an adverse prognosis factor for CRC survival by the College of American Pathologists (33). This feature is mostly found in patients with metastatic disease (34). Ganapathi described vascular invasion in 38% and 28% of colorectal tumors in patients younger and older than 40 years, respectively; and, appointed it as an independent prognostic factor related to survival (16). We reported vascular invasion in 23.5% of cases. Nonetheless, it is worth mentioning that in 25% of our cases, this feature was not included in the pathological report.
The preoperative CEA also has prognostic significance. It has been shown that a ≥5.0 level has an adverse impact on survival. For example, within a series of 17,910 colon cancer patients, an elevated pre operative CEA level was associated with a significant increase on mortality risk, regardless the stage (35). Indeed, some authors suggest that preoperative CEA should be included in the TNM staging (33). Even though 32.7% of our patients presented with increased CEA, this was not a prognostic factor related to survival.
The literature indicates that most of young CRC patients are diagnosed with advanced disease (stage III or IV). In the largest series, advanced disease at diagnosis, ranges between 66% and 81% (14,16,18). Among other authors, you reported significant differences in the proportion of advanced disease between young and over-50-years patients (young patients: 63% and 57% for colon and rectum, respectively; older patients, 49% and 46%, respectively) (7). In concordance, the present study reports that most of our colon cancer patients (63%) were diagnosed with advanced disease, finding T3 or T4 wall invasion in 86% and nodal involvement in 45%. Regarding rectal cancer, the proportion of patients with advanced disease is harder to establish due to the fact that most of the patients (52/90) received NA chemoradiation, and none of them underwent rectal echoendoscopy nor pretreatment MRI. Nonetheless, it is important to mention that as preoperative stage fails to consider the patient’s response to NA treatment, some authors disregard the pre-therapy stage and rely on the response achieved, reflected in the post- treatment pathologic stage (ypTNM) (36). As we know, chemoradiation administered before surgery may alter the pathological N and T, achieving a decrease in tumoral invasion depth and in some cases, even the complete disappearance of malignant cells in the rectal wall and perirectal nodes. This downstaging rate is reported around 60% (37) and complete pathological response (cPR) within a range of 15% to 27% (37-39). In our study, and taking into account only the pathological findings, which include all the patients that underwent surgery, whether it was primarily (pTNM) or after receiving neoadjuvant treatment (ypTNM); 60% of rectal cancer patients were found in stage III or IV. In 55% of cases, the depth of invasion was T3 or T4 and there was nodal involvement in 45% of the cases. In the multivariate analysis, stage was the sole prognostic factor independently related to survival.
Most reports agree that in general, young patients OS is worse than what is reported for the older patients. Nevertheless, this difference is lost when analyzing the results stage by stage, with OS being at least equal to that of older patients (16,18,20,40). This event might be a consequence of young patients being diagnosed in more advanced stages. Our series reports that 5-year OS for young CRC patients was 44%. Such results are below those reported by a SEER-based study (27), in which 5-year OS for general population was estimated in 64%; and, also below the results from other comparative studies, that reported a 5-year OS between 56% and 61% for young patients and, between 61% and 65% for the older ones (18,40). However, and confirming the already-exposed statements, when determining OS stage by stage, we found that results were comparable to the older population. For colon cancer patients, 5-year OS was estimated in 100%, 73%, 55% and 12% for stages I, II, III and IV, respectively. In rectal cancer cases, while OS was estimated in 100% for pathological stages 0 (pCR), I and II; it was only 25% for stage III. It is worth pointing out that almost half of our stage III patients corresponded to the IIIC subcategory for which the OS is described around 33% (21).
In spite of the more aggressive histopathological features, OS by clinical stage appears to be similar between younger and older patients. The performance status in relation with age, probably provides a biological advantage regarding comorbidities, perioperative complications and tolerance to chemo or radiotherapy (10,20). Moreover, young age may have a favorable influence on the interaction between the tumor and the immune system (29). Additionally, microsatellite instability, which is more frequent among the young, is a favorable independent prognostic factor for survival (41).
The lack of a comparative group of older patients within the same institution in order to consistently determine the differences between both groups represents a limitation of the present study. Our future direction, regarding this database, is to molecularly characterize CRC in our young patients.
In conclusion, CRC among young patients tends to occur in the distal colon, or rectum. It exhibits, with more frequency, a high grade, or a mucinous or signet ring cells histology and, likewise, it tends to be diagnosed in more advanced stages. Nonetheless, OS stage by stage is comparable to the one achieved by older patients.
The tendency of young patients to present with advanced and potentially more aggressive disease, should alert the physician to identify the initial clinical manifestations and to expediently assess symptomatic patients.
Competing interests: The authors have declared that no competing interests exist.
Funding: None.
Author contributions: Conceived and designed the idea: RR, PM. Acquisition of data: RR, EFR. Revision of pathologic samples:LT. Analyzed the data: RR, RM. Wrote the first draft of the manuscript: RR. Contributed to the writing of the manuscript: RR, LT, EFR, LC, PM. Agree with manuscript results and conclusions: RR, LT, EFR, RM, LC, PM. All authors have read, and confirm that they meet the Revista Peruana de Gastroenterología criteria for authorship.
REFERENCES
1. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012: Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11; 2013 [Internet]. Geneva: WHO; 2013 [cited 2014 Jul 23]. Available from: http://globocan.iarc.fr [ Links ]
2. Instituto de Enfermedades Neoplásicas (INEN). Registro de Cáncer de Lima Metropolitana; 2013. Lima: INEN;2014 [cited 2014 Jul 23] Available from: http://goo.gl/WsqU5D
3. Atkin WS, Cuzick J, Northover JM, Whynes DK. Prevention of colorectal cancer by once-only sigmoidoscopy. Lancet. 1993;341(8847):736-40. [ Links ]
4. Qaseem A, Denberg TD, Hopkins RH Jr, Humphrey LL, Levine J, Sweet DE, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156(5):378-86.
5. Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1695-8 [ Links ]
6. Meyer JE, Narang T, Schnoll-Sussman FH, Pochapin MB, Christos PJ, Sherr DL. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116(18):4354-9. [ Links ]
7. You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012;172(3):287-9. [ Links ]
8. Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J. The increasing incidence of young- onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89(2):216-24. [ Links ]
9. Cusack JC, Giacco GG, Cleary K, Davidson BS, Izzo F, Skibber J, et al. Survival factors in 186 patients younger than 40 years old with colorectal adenocarcinoma. J Am Coll Surg. 1996;183(2):105-12. [ Links ]
10. O’Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg. 2004;187(3):343-8. [ Links ]
11. Chou CL, Chang SC, Lin TC, Chen WS, Jiang JK, Wang HS, et al. Differences in clinicopathological characteristics of colorectal cancer between younger and elderly patients: an analysis of 322 patients from a single institution. Am J Surg. 2011;202(5):574-82. [ Links ]
12. Luy Lossio G, Maldonado Landa G, Chinga Alayo E, Luy Lossio S, Peinado Rodríguez J. Características clínicas del cáncer colorectal en el Hospital E. Rebagliati Martins 1995-1999. Rev Gastroenterol Peru. 2000;20(4):406-413. [ Links ]
13. Zbuk K, Sidebotham EL, Bleyer A, La Quaglia MP. Colorectal cancer in young adults. Semin Oncol. 2009;36(5):439-50. [ Links ]
14. Dozois EJ, Boardman LA, Suwanthanma W, Limburg PJ, Cima RR, Bakken JL, et al. Young-onset colorectal cancer in patients with no known genetic predisposition: can we increase early recognition and improve outcome? Medicine (Baltimore). 2008;87(5):259-63. [ Links ]
15. Liang JT, Huang KC, Cheng AL, Jeng YM, Wu MS, Wang SM. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg. 2003;90(2):205-14. [ Links ]
16. Ganapathi S, Kumar D, Katsoulas N, Melville D, Hodgson S, Finlayson C, et al. Colorectal cancer in the young: trends, characteristics and outcome. Int J Colorectal Dis. 2011;26(7):927-34. [ Links ]
17. Beckman EN, Gathright JB, Ray JE. A potentially brighter prognosis for colon carcinoma in the third and fourth decades. Cancer. 1984;54(7):1478-81. [ Links ]
18. O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Do young colon cancer patients have worse outcomes?World J Surg. 2004;28(6):558-62. [ Links ]
19. Wang L, Hollenbeak CS, Stewart DB. Node yield and node involvement in young colon cancer patients: is there a difference in cancer survival based on age? J Gastrointest Surg. 2010;14(9):1355-61. [ Links ]
20. Schellerer VS, Merkel S, Schumann SC, Schlabrakowski A, Förtsch T, Schildberg C, et al. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer: CRC in patients under 50 years of age. Int J Colorectal Dis. 2012 Jan;27(1):71-9.
21. American Joint Committee on Cancer. Colon and rectum. En: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7ma ed. New York: Springer; 2010:143-64. [ Links ]
22. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044-58. [ Links ]
23. Castro-Mujica María, Sullcahuamán-Allende Y, Barreda- Bolaños F, Taxa-Rojas LSíndromes hereditarios de predisposición al cáncer colorrectal identificados en el Instituto Nacional de Enfermedades Neoplásicas (INEN), Lima, Perú. Rev Gastroenterol Peru. 2014;34(2):107-14.
24. Cleary J, Peters TJ, Sharp D, Hamilton W. Clinical features of colorectal cancer before emergency presentation: a population-based case-control study. Fam Pract. 2007;24(1):3-6. [ Links ]
25. Iacopetta B. Are there two sides to colorectal cancer? Intt J Cancer. 2002;101(5):403-8. [ Links ]
26. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-17. [ Links ]
27. Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975-2010 [Internet]. Bethesda, MD: National Cancer Institute; 2013 [cited 2014 Jul 23]. Disponible en: http://seer.cancer.gov/csr/1975_2010/
28. Gonzalez EC, Roetzheim RG, Ferrante JM, Campbell R. Predictors of proximal vs distal colorectal cancers. Dis Colon Rectum. 2001;44(2):251-8. [ Links ]
29. Chiang JM, Chen MC, Changchien CR, Chen JS, Tang R, Wang JY, et al. Favorable influence of age on tumor characteristics of sporadic colorectal adenocarcinoma: patients 30 years of age or younger may be a distinct patient group. Dis Colon Rectum. 2003;46(7):904-10. [ Links ]
30. Casavilca Zambrano S, Sanchez Lihon J, Zavaleta A. Carcinoma de células en anillo de sello del colon y recto en el Instituto Nacional de Enfermedades Neoplásicas. Rev Gastroenterol Peru. 2004;24(3):234-7. [ Links ]
31. Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19(9):2814-21. [ Links ]
32. Kang H, O’Connell JB, Maggard MA, Sack J, Ko CY. A 10- year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48(6):1161-8. [ Links ]
33. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124(7):979-94. [ Links ]
34. Lui K, Enjoi K, Inokuchi K. Venous permeation of colorrectal carcinoma. Jpn J Surg. 1980;10(4):284-9. [ Links ]
35. Thirunavukarasu P, Sukumar S, Sathaiah M, Mahan M, Pragatheeshwar KD, Pingpank JF, et al. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011;103(8):689-97. [ Links ]
36. Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Saltz LB, et al. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer. 2008;113(1):57-64. [ Links ]
37. Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44(5):1027-38. [ Links ]
38. Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27(31):5124-30. [ Links ]
39. Shivnani AT, Small W Jr, Stryker SJ, Kiel KD, Lim S, Halverson AL, et al. Preoperative chemoradiation for rectal cancer: results of multimodality management and analysis of prognostic factors. Am J Surg. 2007;193(3):389-93.
40. Yeo SA, Chew MH, Koh PK, Tang CL. Young colorectal carcinoma patients do not have a poorer prognosis: a comparative review of 2,426 cases. Tech Coloproctol. 2013;17(6):653-61. [ Links ]
41. Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342(2):69-77. [ Links ]
Corresponding Author: Rossana Ruiz
Av. Angamos Este 2520, Surquillo, Lima, Perú.
E-mail: rossana_rm@hotmail.com
Recibido: 23-07-2015
Aprobado: 29-09-2015