Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Gastroenterología del Perú
versión impresa ISSN 1022-5129
Rev. gastroenterol. Perú vol.38 no.1 Lima ene./mar. 2018
ARTÍCULOS ORIGINALES
Oncological outcomes in extended time intervals between preoperative chemoradiotherapy with capecitabine and surgery in operable rectal adenocarcinoma
Resultados oncológicos en intervalos extendidos de tiempo entre quimioradioterapia preoperatoria con capecitabina y cirugía en adenocarcinoma rectal operable
Miguel A. Muñoz1,a, Manuel G. Palacios1,a, Jenny Malca2,a, Raúl Mantilla3,b, Paola C. Montenegro4,c, Ivan Chavez5,d
1 Department of Oncologic Surgery, Instituto Nacional de Enfermedades Neoplasicas. Lima, Peru.
2 Department of Radiation Therapy, Instituto Nacional de Enfermedades Neoplasicas. Lima, Peru.
3 Instituto Nacional de Enfermedades Neoplasicas. Lima, Peru.
4 Department of Oncology Medicine, Instituto Nacional de Enfermedades Neoplasicas. Lima, Peru.
5 Department of Abdominal Surgery, Instituto Nacional de Enfermedades Neoplasicas. Lima, Peru.
a Resident, b Bachelor in Biostatistics, c Assistant, d Institutional director
ABSTRACT
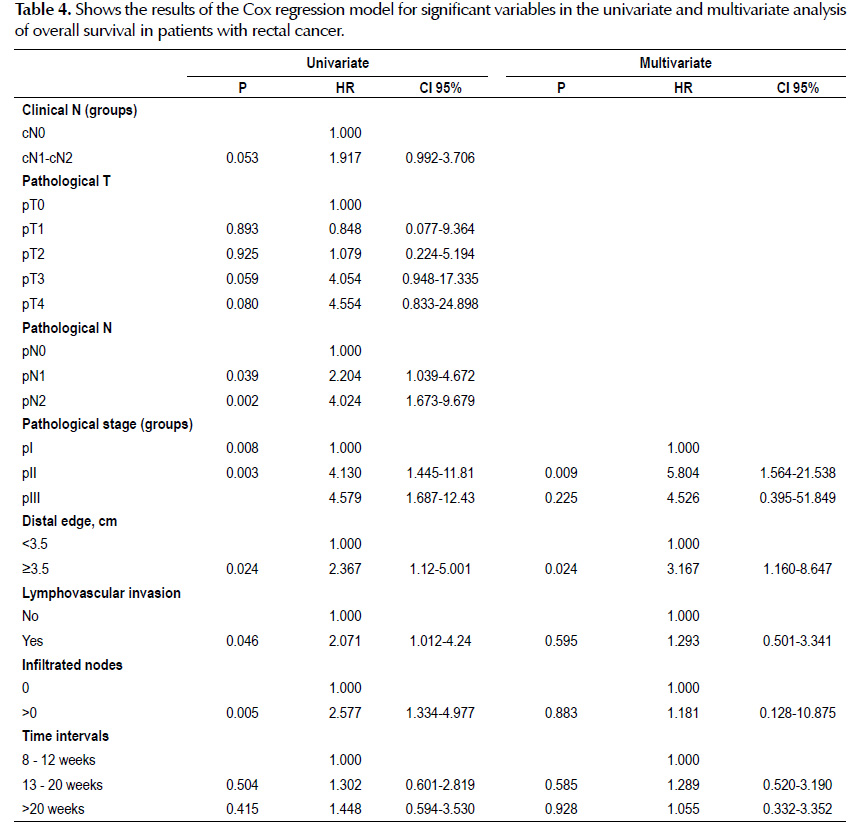
Objective: To assess whether extended time intervals (8-12, 13-20 and >20 weeks) between the end of neoadjuvant chemoradiotherapy and surgery affect overall survival, disease-free survival. Materials and methods: Retrospective study in 120 patients with rectal adenocarcinoma without evidence of metastasis (T1-4/N0-2/M0) at the time of diagnosis that underwent surgery with curative intent after neoadjuvant chemoradiotherapy with capecitabine and obtained R0 or R1 resection between January 2010 to December 2014 at the National Cancer Institute of Peru. Dates were evaluated by Kaplan-Meier method, log- rank test and Cox regression analysis. Results: Of the 120 patients, 70 were women (58%). The median age was 63(26-85) years. All received neoadjuvant chemoradiotherapy. No significant difference was found between the association of the median radial (0.6, 0.7 and 0.8 cm; p=0.826) and distal edge (3.0, 3.5 and 4.0 cm; p=0.606) with time interval groups and similarly the mean resected (18.8, 19.1 and 16.0; p=0.239) and infiltrated nodules (1.05, 1.29 and 0.41); p=0.585). The median follow-up time of overall survival and desease free survival was 40 and 37 months, respectively. No significant differences were observed in overall survival (79.0%, 74.6% and 71.1%; p=0.66) and disease-free survival (73.7%, 68.1% and 73.6%; p=0.922) according to the three groups studied at the 3-year of follow-up. Conclusions: We found that widening the time intervals between the end of neoadjuvant chemoradiotherapy and surgery at 24 weeks does not affect the overall survival, disease-free survival and pathological outcomes. It allows to extend the intervals of time for future studies that finally will define the best time interval for the surgery.
Keywords: Neoadjuvant treatment; Chemoradiotherapy; Rectal cancer; Time Intervals (source: MeSH NLM).
RESUMEN
Objetivo: Evaluar si los intervalos de tiempo extendidos (8-12, 13-20 y >20 semanas) entre el fin de la quimioradioterapia neoadyuvante y la cirugía afectan la sobrevida global, y la sobrevida libre de enfermedad. Material y métodos: Estudio retrospectivo de 120 pacientes con adenocarcinoma rectal sin evidencia de metástasis (T1-4/N0-2/M0) al momento del diagnóstico que se sometieron a cirugía con intención curativa luego de quimioradioterapia neoadyuvante con capecitabina y tuvieron resección R0 o R1 entre enero 2010 y diciembre 2014 en el Instituto Nacioanal de Enfermedades Neoplásicas de Perú. El análisis se hizo con el método de Kaplan-Meier, la prueba log-rank y la regresión de Cox. Resultados: De 120 pacientes, 70 fueron mujeres (58%). La mediana de la edad fue 63 años (26-85 años). Todos recibieron quimioradioterapia neoadyuvante. No hubo diferencia significativa entre la asociación de las medianas de los bordes radial (0,6, 0.7 y 0,8 cm; p=0,826) y distal (3,0, 3,5 y 4,0 cm; p=0,606) con los intervalos de tiempo de los grupos y similarmente con la media de los ganglios resecados (18,8, 19,1 y 16,0; p=0,239) e infiltrados (1,05, 1,29 y 0,41; p=0,585). No se observaron diferencias significativas en sobrevida global (79,0%, 74,6% y 71,1%; p=0,66) y sobrevida libre de enfermedad (73,7%, 68,1% y 73,6%; p=0,922), en los tres grupos estudiados a 3 años de seguimiento. Conclusiones: Encontramos que aumentar los intervalos de tiempo entre el fin de la quimioradioterapia neoadyuvante y la cirugía hasta 24 semanas no afecta la sobrevida global, sobrevida libre de enfermedad ni los desenlaces patológicos. Esto permitiría extender los intervalos de tiempo en estudios futuros para definir el mejor intervalo de tiempo para la cirugía.
Palabras clave: Tratamiento neoadyuvante; Quimioradioterapia; Cáncer del recto (fuente: DeCS BIREME).
INTRODUCTION
In the world, colorectal cancer ranks third in frequency in men (746,000 cases, 10.0% of the total) and the second in women (614,000 cases, 9.2% of the total) (1). In Peru, it is the fourth most frequent in men (standardized rate for the age of 10.2 per. 100000) representing 7.2% of all the cancers (1318 cases). In women it is also the fourth in frequency (ASR 11.9 per 100,000) representing 7.1% of all cancers (1735 cases) (2). In the 2010-2012 period, 1046 new cases of rectum cancer were diagnosed among residents of Metropolitan Lima, with an age-standardized incidence rate of 3.8 cases per 100,000 inhabitants (3).
Korean group determined that the optimal period of time to perform surgery with curative intention in patients with response to neoadyuvant neoadjuvant chemoradiotherapy (CRT) is between 7 to 10 weeks (4). Probst, concluded that performing the surgery after 8 weeks increased the possibility of pathologic complete response (pCR) without association with the increase of surgical complications compared to the interval 6 - 8 weeks (5). Rombouts, reports that there is no difference in the rate of pCR obtained in patients with early tumor stages evaluated at intervals ranging from 5-14 weeks, but in patients with locally advanced disease the interval of 9-12 weeks between neoadyuvant CRT and surgery improves pCR rate with no effect on overall survival (6). Petrelli, reports in a meta-analysis that the pCR rate increases by 6%, if the surgery is performed in a greater interval of 6-8 weeks, with similar results and rate of complications (7). Maas M. reports that patients with pCR have better oncologic outcomes than those who do not (8). There are few studies evaluating cancer outcomes after 14 weeks and the closest reported by Habr-Gamma determined that delaying surgical resection after neoadjuvant CRT for the distal rectum did not increase the risk of relapse of the disease or affect survival but this study does not consider patients with clinical complete response (cCR) (9).
Pathologic complete response (pCR) is defined as the complete absence of intact tumor cells in the resected specimen of patients with neoadjuvant CRT (10).
The primary objective of this study was to assess whether extended time intervals (8-12, 13-20 and >20 weeks) between neoadjuvant CRT and surgery affect overall survival (OS) and disease-free survival (DFS). The secondary objective is to assess the same association with clinicopathological variables.
MATERIALS AND METHODS
Ethics statement
This study was approved by the Institutional Committee on Ethics and Research of Instituto Nacional de Enfermedades Neoplasicas (INEN) (Protocol Number #INEN17-07).
Data source
Medical records of patients with rectal cancer who were operated in the Department of Abdominal Surgery at the INEN between January 2010 to December 2014.
Patient population
Between January 2010 to December 2014, a total of 336 patients with rectal cancer underwent surgery with curative intent in the Department of Abdominal Surgery at the INEN. From this initial cohort, we selected a series of 120 patients with rectal adenocarcinoma without evidence of metastasis (T1-4/N0-2/M0) according to the American Joint Committee on Cancer Staging (AJCC) manual seventh edition, at the time of diagnosis; Who underwent surgery with curative intent after neoadjuvant CRT with capecitabine and obtained R0 or R1 resection. Patients undergoing emergency surgery, patients with R2 surgery, different histology of adenocarcinoma, neoadjuvant CRT with intravenous 5-FU, neoadjuvant CRT in other institution, incomplite treatment with capecitabine and underwent surgery before 8 weeks have been excluded. We got 6 patients with R2 resection and 4 who underwent surgery before 8 weeks. Patients were categorized into 3 groups based on time intervals (8-12, 13-20 and >20 weeks) between the end of neoadjuvant CRT and the day of surgery. The only reason for delay in surgery was hospital bed availability. Demographic, clinical and pathological data were collected on a collection sheet.
Study variables
Time interval between the end of neoadjuvant CRT and surgery was the variable of interest, which included 8-12,13-20 and >20 weeks. The first interval was chosen to compare with previous studies and the others by author's choice because these intervals were not evaluated in previous studies.
Other variables used in this study included overall survival, disease-free survival, demographic variables, tumor clinical and pathological characteristics. Demographic variables included age and sex. Tumor clinical characteristics included pretreatment carcinoembryonic antigen (CEA) level, clinical T, clinical N, clinical stage, location by colonoscopy and distance from the tumor to anal verge. Tumor pathological characteristics included, histological type, tumor pathological response, pathological T, pathological N, pathological stage, proximal, radial and distal edge, histological grade, limphovascular invasion, perineural invasion, type of resection, preservation sphincter rate, compromised and resected nodes.
Tumor clinical and pathological stage was determined according to the American Joint Committee on Cancer staging manual seventh edition. The primary objectives of the study were to assess whether extended time intervals (8-12, 13-20 and >20 weeks) between neoadjuvant CRT and surgery improve OS and DFS.
Evaluation
For diagnosis and clinical workup, history taking and digital rectal examination were done and complete blood counts, blood chemistry for liver and kidney functions, and CEA level were checked. Imaging studies included abdomen and pelvis computed tomography (CT), chest radiography and colonoscopy with biopsy in all cases, only in some cases include pelvic MRI and chest CT. All patients had a performance status of one according to the WHO, prior to treatment.
Neoadjuvant CRT
These regimens consisted of radiotherapy (RT) concomitant with chemotherapy (QT); which in all cases was capecitabine at doses of 825 mg/m2 orally 5 or 7 days per week (twice per day) during RT. The radiotherapy technique was external RT in pelvic fields, with a 4-field box technique at 45 Gy doses in 25 sessions, with 180 cGy / day + boost 540 (total dose 5,040 cGy), five days a week, for five weeks concurrent to QT. In some cases, the doses of RT were individualized. Seven neoadjuvant regimens were applied, the most frequent being 5040 cGy/28 sessions + QT in 94 patients (78.3%), and the other were 6,600 cGy/36, 5,400 cGy/30, 5,000 cGy/25, 6,000 cGy/32, 4,500 cGy/25 and 3,900 cGy/13 sessions in 1 (0.8%), 8 (6.7%), 3 (2.5%), 1 (0.8%), 12 (10.0%) and 1 (0.8%), respectively.
Surgery
All patients underwent pelvic examination under anesthesia on the day of surgery and some of them prior to surgery. The procedures included abdominoperineal resection (APR), low anterior resection (LAR), ultra-low anterior resection (ULAR), local resection (LR), pelvic exenteration (PE) and Hartmann´s procedure (HP) in 49.2% (n=59), 29.2% (n=35), 16.7% (n=20), 2.5% (n=3), 0,8% (n=1) and 1.7% (n=2) respectively. All patients received total mesorectal excision, except LR. Conventional open surgery were performed in 106 patients (88.3%), laparoscopic approach in 11 (9.1%) and transanal resection in 3 (2.5%). The mean interval time between neoadjuvant CRT and surgery was 17.86 weeks (range, 8-160). Patients with pathological stage III (pTx/N1–2/M0) were referred to a medical oncologist for adjuvant therapy. Adjuvant chemotherapy was required in 34 patients (28.3%) after surgery.
Patients' follow-up
The patients underwent a regular checkup every 3-4 month during the first 2 years after leaving the hospital, then every 6 months during the third and fourth year and finally every twelve months after the fifth year. The controls include rectal examination, CEA serum level, abdominal ultrasound and chest radiographs. Control colonoscopy and additional imaging exams to rule out distant metastasis were done by the clinician's decision. All patients who do not attend to their scheduled follow-up are considered lost. Patients were followed until December 2016, the date on which the number of deceased patients in our sample was determined, checking the data with those of the National Registry of Identification and Civil Status (RENIEC). So, in the end we had 36 dead, 82 alive and 2 lost control.
Statistical analysis
Continuous variables were represented by means, medians and range, and categorical variables with frequencies and percentages. The analysis between the variable of interest with continuous variables, were developed using the ANOVA or Kruskal Wallis test; And Chi-square test to associate with categorical variables, as well as categories of these variables were grouped in the case that was considered necessary. Kaplan-Meier method, log-rank test and Cox Regression Analysis were used to determine the relationship between possible significant risk factor for overall survival (OS) and disease-free survival (DFS). Multivariate data analysis includes only the factors identified as significant in univariate analysis and the variable of interest was include in both analysis. OS was defined as the period between surgery to death, DFS was defined as the time between surgery to recurrence and patients who did not die or who did not recur were censored. OS was calculated using the number of deaths prior to December 31, 2016. Differences were considered significant if p<0.05. The data were evaluated using SPSS v. 22 (11,12).
Pathology report
Pathologic specimens were evaluated using the standardized protocol of colon-rectum of the pathology department of INEN, based on protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum of the College of American Pathologists and this instrument is used since the last months of 2012 that is the reason why we could not get the tumor pathological response grade of 36 patients (29.0%), but we are sure that they did not get pCR because pathologic report confirm adenocarcinoma. Pathological complete response (pCR) is the same as tumor pathological response grade 0 in our study.
RESULTS
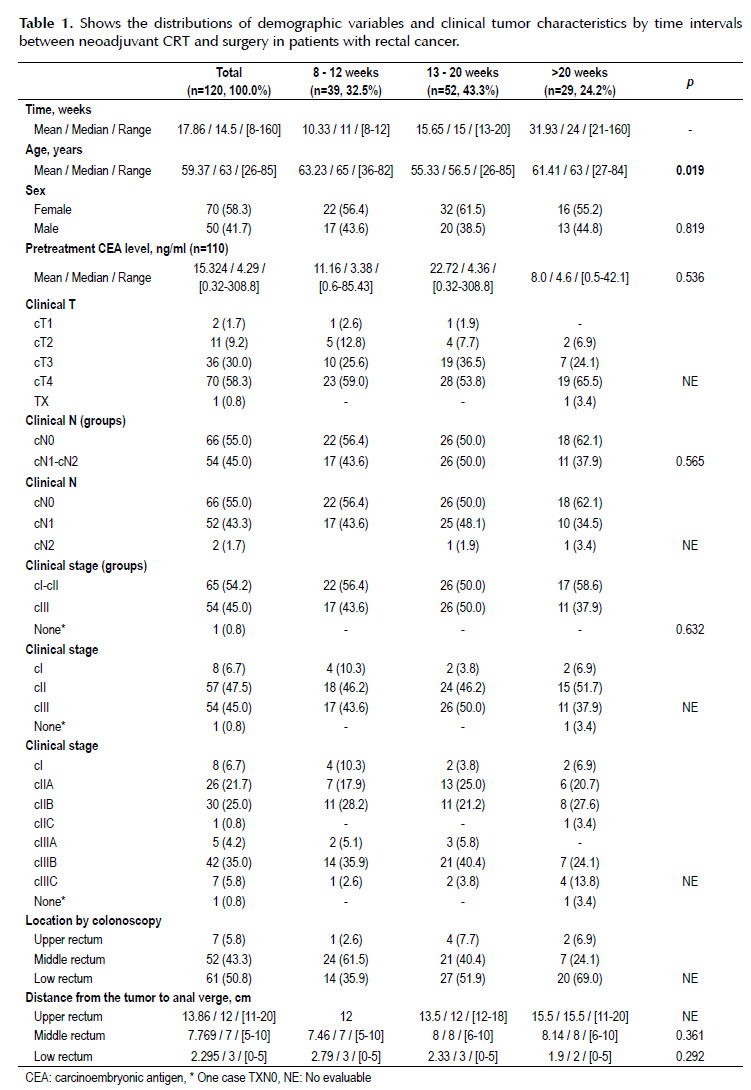
The mean pretreatment CEA level was 15.3 ng/ml (range 0.32-308.8). The most frequent clinical stage was cT4 (58.3%), cN0 (55%) and IIIB (35%). Fifty-one percent were localized in low rectum and the mean distance from the tumor to anal verge was 2.3 cm (range 0-5 cm) (Table 1).
There is significant difference between the mean ages of the three groups (63; 55 and 61 years; p=0.019). The association between clinical T, tumor location by colonoscopy and the mean distance from the upper rectum tumor to anal verge in the three time intervals groups could not be assessed because sample size was small for this stratified analyses. The median time interval between neoadjuvant CRT and surgery in the three groups (8-12, 13-20 and >20 weeks) was 11, 15 and 24 weeks respectively (Table 1).
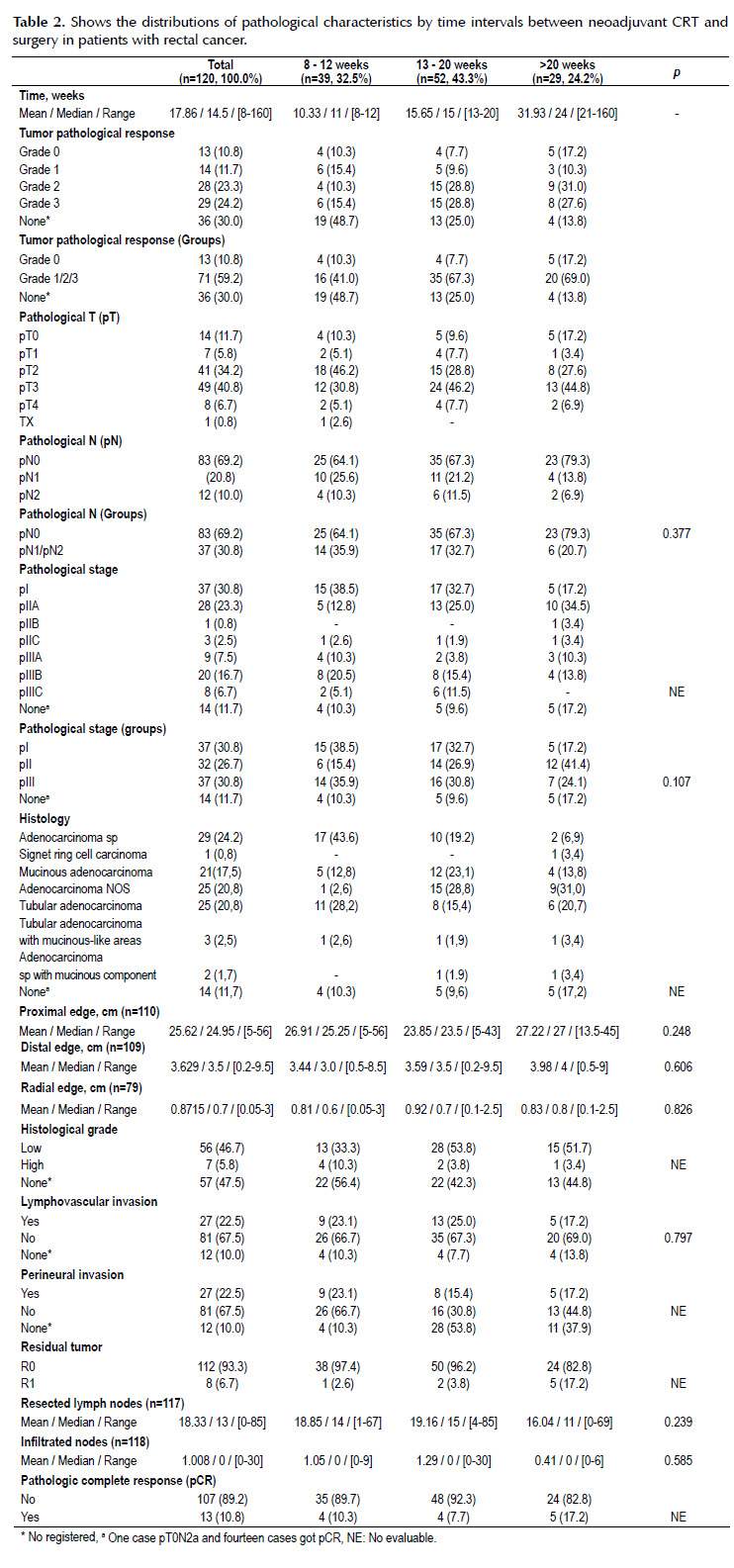
There is not significant difference between pathological variables on the three intervals groups. The association between tumor pathological response, pathological T, histological type and grade, perineural invasion, residual tumor and pCR status with time intervals groups could not be assessed because sample size were small for this stratified analyses (Table 2).
The pCR rate in all patients were 10.8% (n=13) and the incidence was 10.3% (n=4), 7.7% (n=4) and 17.2% (n=5) in time interval groups (8-12, 13- 20 and >20 weeks) respectively. The most frequent pathological stage was pT3 (40.8%), pN0 (69.2%) and III (30.8%). The predominant histological type was adenocarcinoma sp (24.2%). The average radial and distal edge was 0.87 cm (range 0.05-3 cm) and 3.62 cm (range 0.2-9.5 cm) respectively. High histological grade, lymphovascular invasion and perineural invasion was found in 5.8% (n=7), 22.5% (n=27) and 11.7% (n=14) respectively. Only 6.7% (n=8) has R1 resection. The average resected and infiltrated lymph nodes was 18.3 (range 0-85) and 1.0 (range 0-30) respectively (Table 2).
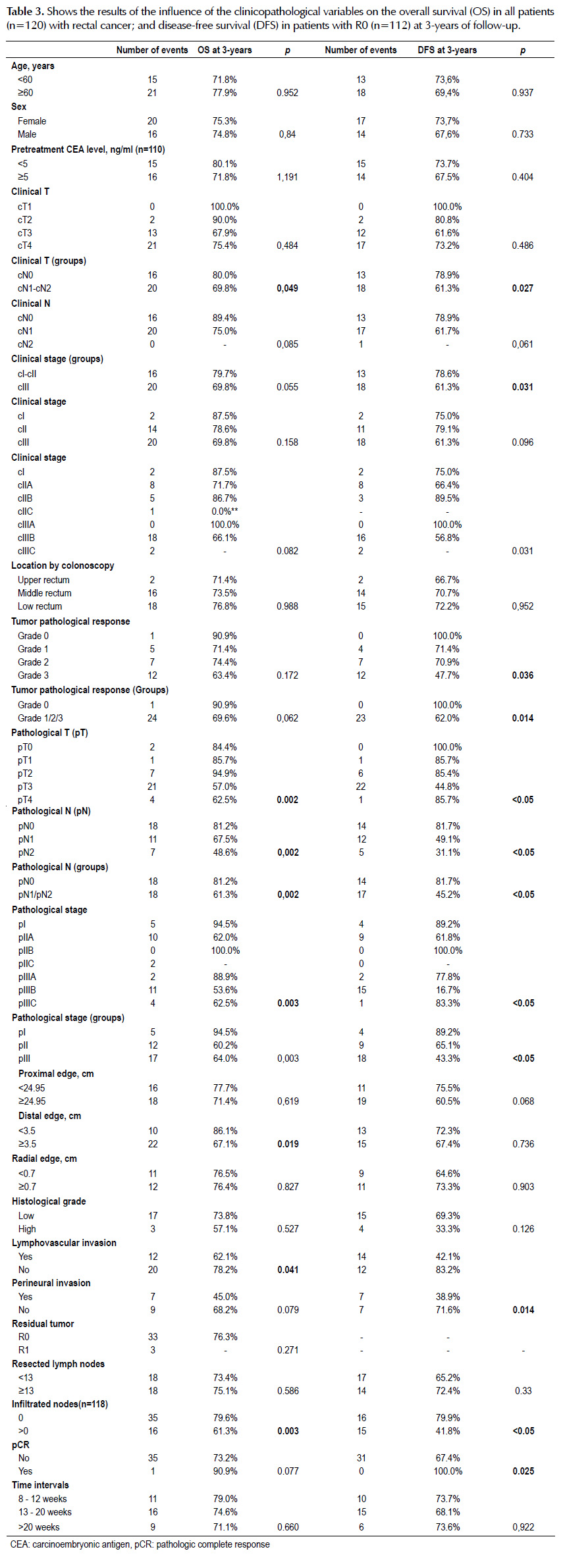
The clinical N (p=0.049), pathological N (p=0.002), pathological stage by groups (p=003), distal edge (p=0.002), lymphovascular invasion (p=0.041), distal edge (p=0.019) and infiltrated nodes (p=0.003) were the variables significantly associated with overall survival (Table 3).
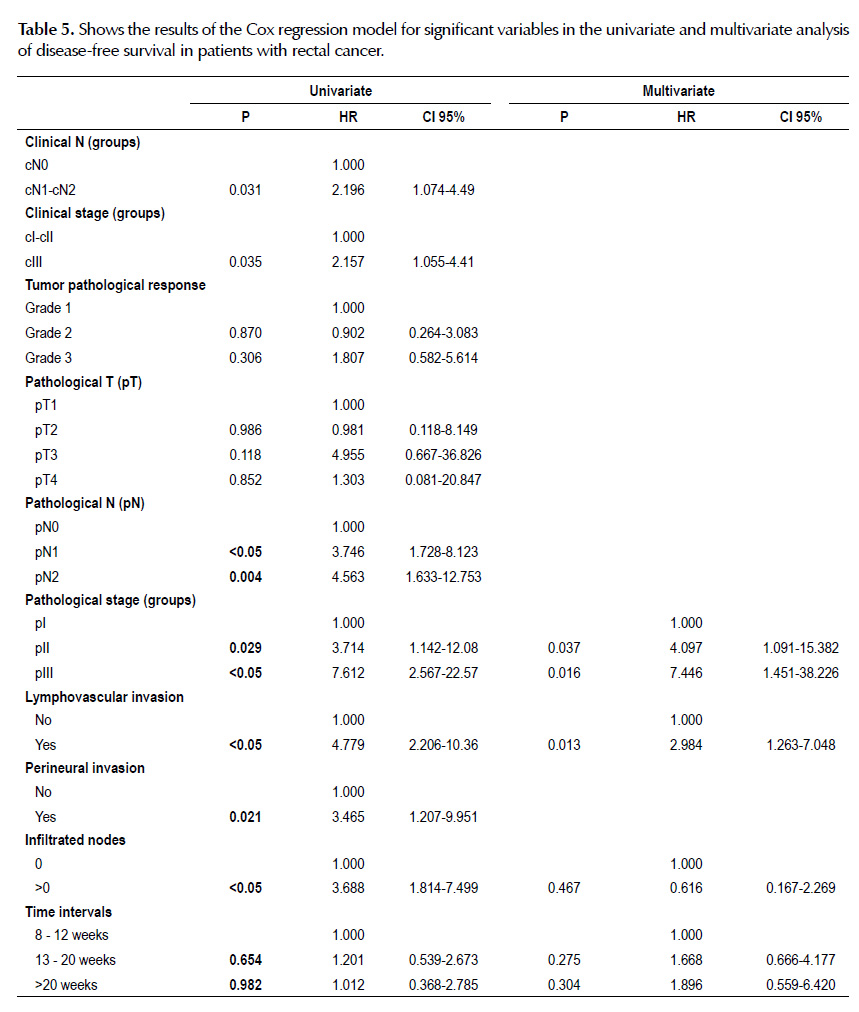
Clinical N by groups (p=0.027), clinical stage by groups (p=0.031), tumor pathological response (p=0.036), tumor pathological response by groups (p=0.014), pathological T (p<0.05), pathological N (p<0.05), pathological stage by groups (p<0.05), lymphovascular invasion (p<0.05), perineural invasion (p=0.014), infiltrated nodes (p<0.05) and pathologic complete response (p=0.025) were the variables significantly associated with disease-free survival (Table 3).
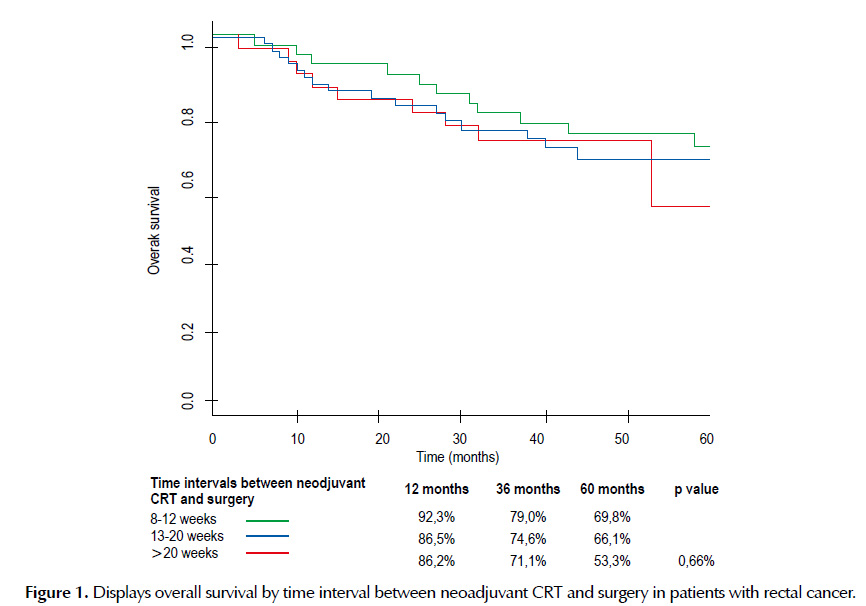
There is not significant difference (p=0.66) in overall survival between the three groups of time intervals between neoadjuvant CRT and surgery at 3-year of follow-up (Table 3). The median OS follow-up time was 40 months and no significant differences were observed according to the three groups studied at the 5-year of follow-up (Figure 1).
There is not significant difference (p=0.922) in disease-free survival in patients with R0 between the three groups of time intervals between neoadjuvant CRT and surgery at 3-year of follow-up (Table 3). The median DFS follow-up time was 37 months no significant differences were observed according to the three groups studied at 5-year of follow-up. (Figure 2).
In the univariate analysis, variables associated with a higher likelihood of overall survival were lower pathological N (p=0.039), lower pathological stage by groups (p=0.008), distal edge ≥3.5 cm (p=0.024), without lymphovascular invasion (p=0.046) and without infiltrated nodes (p=0.005). On multivariable analysis, lower tumor pathological stage by groups (p=0.009) and distal edge ≥3.5 cm (p=0.024) remained significant prognostic factors of overall survival (Table 4).
In the univariate analysis, variables associated with a higher likelihood of disease-free survival were lower clinical N by groups (p=0.031), lower clinical stage by groups (p=0.035), lower pathological N (p<0.05), lower pathological stage by groups (p=0.029), without lymphovascular invasion (p<0.05), without perineural invasion (p=0.021) and without infiltrated nodes (p<0.05). On multivariable analysis, lower tumor pathological stage by groups (p=0.037) and without lymphovascular invasion (p=0.013) remained significant prognostic factors of disease-free survival (Table 5).
DISCUSSION
Our study examined whether long-term (8-12, 13-20 and >20 weeks) intervals between the end of neoadjuvant CRT and surgery affect overall survival (OS) and disease-free survival(DFS). Our results suggest that the OS and DFS are not affected by extending the time intervals. The univariate and multivariate analysis of the variable time interval shows that it is not a prognostic factor for OS and DFS. On multivariable analysis we determined that lower tumor pathological stage by groups and distal edge are independent prognostic factor of OS and tumor pathological stage by groups and lymphovascular invasion are independent prognostic factor of DFS.
We found that the highest OS and DFS at 3 years of follow-up was 79% and 73.7%, respectively. Our findings have similar outcomes with the studies reported by Wang, reported a 3-year OS rate of 92% and a DFS of 76% (13). Sauer, reported an OS at 5 years of 76% and DFS of 68% in the group with neoadyuvant CRT prior to surgery (14). Krishnan, reported a 2 years OS and DFS rate of 98% and 76% respectively (15). Chan, reported a 3-year OS rate of 86% with a median follow-up time of 2.3 years in the group receiving RT and capecitabine (16).
The pCR rate in studies in which capecitabine was used as concurrent chemotherapy varies from (10.5% - 27%) reported by De Paoli, Krishnan, kim, korkolib, Conde, kocakova and Wong (10,15,17-19). The rate of pCR in all patients of our study is 10.8% that is into the range. The pCR rate in one of the largest studies was 13.5% reported by Hartley (10). Our study is the first study in which the pCR rate is reported at time intervals between [13-20 weeks] and [>20 weeks].
Other oncological outcomes analyzed were the association between radial and distal edge with time intervals, and no significant differences were found. The mean distal edge in our study was 3.62 cm [0.2-9.5 cm] and distal edge ≥3.5 cm (p=0.024) resulted an independent prognostic factor of overall survival in the multivariate analysis. The literature reports that distal margin of 2 cm is suitable for most rectal cancers. The mean radial edge in our study was 0.87 cm [0.05-3] and we know that radial edge of 1 mm have a high risk of distant metastases (37.6 vs 12.7%, p=0001) (20).
No significant differences were found in the association between resected and compromised nodes with time interval groups, but the observation of interest is that the mean of the resected and compromised nodes decreases if we extend the time interval until surgery. That point requires further investigation. The mean number of resected and compromised nodes in all our patients was 18.3 and 1.0 respectively. These findings are similar to those reported by Codina Cazador in 162 patients, with an average of resected and compromised nodes of 19.6±11.8 and 0.6±1.9 respectively (21). Wichmann, reported in 42 patients, an average of 13.6 and 1.4 nodes, respectively (22). The AJCC and UICC recommend at least 12 lymph nodes in the surgical specimen to confirm lymph node staging (23). Our patients had a pathological lymph node involvement of 30.8% (37 of 120). Rivas reported 47.6% (10 of 21) (24). Chan reported 44% (15 of 34) with concurrent RT + capecitabine (16). Kim reports 38.7% (48 of 124) with concurrent RT + capecitabine (25).
Sauer reports in a randomized study in which patients requiring abdominoperineal resection at baseline after neoadjuvant CRT + surgery achieved a higher rate of sphincter preservation than those who did not receive CRT prior to surgery (39% vs. 19%, p=0.004) (14). Our sphincter preservation rate in patients with tumor located in the lower rectum by colonoscopy (≤5 cm of anal verge) was 29.5% (18 of 61 patients). Similar results were reported by De Bruin, reports a rate of 25% (8 of 32) of distal rectal tumors, where LAR was performed (26). CHAN, reports a rate of 23% (6 of 26) after capecitabine + RT and 31% (16 of 52) after 5-FU, leucovorin, mitomycin + RT in tumors 7 cm from the anal margin (16). Fernández- Martos reports a rate of 25% (11 of 43) in patients with tumors 2 cm from the anal margin that would initially be submitted to APR (27).
These findings have important clinical and logistic implications, especially in state institutes with a high degree of specialization and a large number of patients such as ours. The present study is the only one that reports these results in time intervals beyond 14 weeks and it allows to extend the intervals of time between the end of neoadjuvant CRT and surgery for future studies. These future studies would ultimately define the best time interval for surgery.
In conclusion, we found that amplifying the time intervals between the end of neoadjuvant chemoradiotherapy and surgery at 24 weeks does not affect the overall survival, disease-free survival and pathological outcomes. The present study is the only one that reports these results at these time intervals. Our pathologic and survival outcomes in these amplified intervals are within the range reported in the time intervals recommended by the world literature. It allows to extend the intervals of time for future studies that finally will define the best time interval for the surgery.
Conflict of interest: None of the authors has any conflict of interest.
BIBLIOGRAPHIC REFERENCES
1. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012: Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11; 2013 [Internet]. Geneva: WHO; 2013 [cited 2014 Jul 23]. Available from: http://globocan.iarc.fr [ Links ]
2. Departamento de Epidemiología y Estadística del Cáncer, Instituto de Enfermedades Neoplásicas (INEN). Cáncer de recto [Internet]. In: Instituto de Enfermedades Neoplásicas. Registro de cáncer de Lima Metropolitana. Estudio de incidencia y mortalidad 2004 - 2005. Lima: INEN; 2014 [cited 2017 May 2]. Available from: http://www.inen.sld.pe/portal/documentos/pdf/banners_2014/Febrero/13022014_Libro_RCLM_04_05.pdf [ Links ]
3. Departamento de Epidemiología y Estadística del Cáncer, Instituto de Enfermedades Neoplásicas (INEN). Cáncer de recto. Registro de cáncer de Lima Metropolitana. Incidencia y nortalidad 2010 - 2012. Lima: INEN; 2014 [cited 2017 May 2]. Available from: http://www.inen.sld.pe/portal/documentos/pdf/banners_2014/2016/Registro%20de%20C%C3%A1ncer%20Lima%20Metropolitana%202010%20-%202012_02092016.pdf [ Links ]
4. Kwak YK, Kim K, Lee JH, Kim SH, Cho HM, Kim DY, et al. Timely tumor response analysis after preoperative chemoradiotherapy and curative surgery in locally advanced rectal cancer: A multi- institutional study for optimal surgical timing in rectal cancer. Radiother Oncol. 2016;119(3):512-8. [ Links ]
5. Probst CP, Becerra AZ, Aquina CT, Tejani MA, Wexner SD, Garcia-Aguilar J, et al. Extended intervals after neoadjuvant therapy in locally advanced rectal cancer: the key to improved tumor response and potential organ preservation. J Am Coll Surg. 2015;221(2):430-40. [ Links ]
6. Rombouts AJM, Hugen N, Elferink MAG, Nagtegaal ID, de Wilt JHW. Treatment interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer patients: a population-based study. Ann Surg Oncol. 2016;23(11):3593-601. [ Links ]
7. Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer a meta-analysis of published studies. Ann Surg. 2016;263(3):458-64. [ Links ]
8. Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835-44. [ Links ]
9. Habr-Gama A, Oliva Perez R, Proscurshim I, Nunes Dos Santos RM, Kiss D, Gama-Rodrigues J, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys. 2008;71(4):1181-8. [ Links ]
10. Hartley A, Ho KF, McConkey C, Geh JI. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br J Radiol. 2005;78(934):934-8. [ Links ]
11. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-81. [ Links ]
12. Cox, David R. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187-220. [ Links ]
13. Wang LW, Yang SH, Lin JK, Lin TC, Chan WK, Chen WS, et al. Pre-operative chemoradiotherapy with oral tegafur- uracil and leucovorin for rectal cancer. J Surg Oncol. 2005;89(4):256-63. [ Links ]
14. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731-40. [ Links ]
15. Krishnan S, Janjan NA, Skibber JM, Rodriguez-Bigas MA, Wolff RA, Das P, et al. Phase II study of capecitabine (xeloda®) andconcomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66(3):762-71.
16. Chan AK, Wong AO, Jenken DA. Preoperative capecitabine and pelvic radiation in Locally advanced rectal cancer—is it equivalent to 5-fu infusion plus leucovorin and radiotherapy? Int J Radiat Oncol Biol Phys. 2010;76(5):1413-9. [ Links ]
17. Conde S, Borrego M, Teixeira T, Teixeira R, Sá A, Soares P. Comparison of neoadjuvant oral chemotherapy with UFT plus Folinic acid or Capecitabine concomitant with radiotherapy on locally advanced rectal cancer. Rep Pract Oncol Radiother. 2012;17(6):376-83. [ Links ]
18. De Paoli A, Chiara S, Luppi G, Friso ML, Beretta GD, Del Prete S, et al. Capecitabine in combination with preoperative radiation therapy in locally advanced, resectable, rectal cancer: a multicentric phase II study. Ann Oncol. 2006;17(2):246-51. [ Links ]
19. Kim JC, Kim T, Kim JH, Yu CS, Kim HC, Chang HM, et al. Preoperative concurrent radiotherapy with capecitabine before total mesorectal excision in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2005;63(2):346-53. [ Links ]
20. Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Buie WD, et al. Practice Parameters for the management of rectal cancer (revised). Dis Colon Rectum. 2013;56(5):535-50. [ Links ]
21. Codina Cazador A, Farres Coll R, Olivet Pujol F, Grillo AM, Pujadas de Palol M, Gómez Romeua N, et al. [Clinical and oncological results of the pathological complete response in rectal cancer after neoadjuvant treatment]. Cir Esp. 2013;91(7):417-23. Spanish. [ Links ]
22. Wichmann MW, Müller C, Meyer G, Strauss T, Hornung HM, Lau-Werner U, et al. Effect of preoperative radiochemotherapy on lymph node retrieval after resection of rectal cancer. Arch Surg. 2002;137(2):206-10. [ Links ]
23. American College of Surgeons. National Quality Forum Endorsed Commission on Cancer Measures for Quality of Cancer Care for Breast and Colorectal Cancers [Internet]. Chicago, IL: ACS Press Release; May 14, 2007 [cited 1 Feb 2015]. Available from: www.facs.org/cancer/qualitymeasures.html [ Links ]
24. Rivas G, Olivella F, Carreño J, Rodríguez V. Pacientes con cáncer de recto localmente avanzado en tratamiento neoadyuvante con quimiorradioterapia en el Instituto Nacional de Cancerología durante 2010: Rev Colomb Cancerol. 2013;17(1):18-24. [ Links ]
25. Kim DY, Jung K H, Kim TH, Kim DW, Chang HJ, Jeong JY, et al. Comparison of 5-fluorouracil/leucovorin and capecitabine inpreoperative chemoradiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2007;67(2):378-84. [ Links ]
26. de Bruin AF, Nuyttens JJ, Ferenschild FT, Planting AS, Verhoef C, de Wilt JH. Preoperative chemoradiation with capecitabine in locally advanced rectal cancer. Neth J Med. 2008;66(2):71-6. [ Links ]
27. Fernandez-Martos C, AparicioJ, Bosch C, Torregrosa M, Campos JM, Garcera S, et al. Preoperative uracil, tegafur, and concomitant radiotherapy in operable rectal cancer: a phase ii multicenter study with 3 years’ follow-Up. J Clin Oncol. 2004;22(15):3016-22.
Correspondence:
Miguel A. Muñoz
Instituto Nacional de Enfermedades Neoplásicas
Narciso de la Colina 1224 Dpto. 602 Barrio médico Surquillo. Lima, Perú.
E-mail: miguel_0074@hotmail.com
Recibido: 23-6-2017
Aprobado: 17-1-2018