Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Gastroenterología del Perú
versión impresa ISSN 1022-5129
Rev. gastroenterol. Perú vol.38 no.1 Lima ene./mar. 2018
ARTÍCULOS ESPECIALES
Ex Vivos Models to Teaching Therapeutic Endoscopic Ultrasound (T-EUS)
Modelos ex-vivo en aprendizaje de la ecoendoscopia terapéutica (EE-T)
Everson L.A. Artifon1, Spencer Cheng1, Thaisa Nakadomari2, Leandro Kashiwagi2, Jose Celso Ardengh3, Emilio Belmonte4,5, Jose P. Otoch1
1 Department of Surgery, University of São Paulo Faculty of Medicine. São Paulo, Brazil.
2 Department of Surgery and Endoscopy, Cajuru University Hospital. Curitiba, Brazil.
3 Department of Surgery and Anatomy, University of São Paulo. Ribeirão Preto, Brazil.
4 Fundação Pio XII, Hospital de Câncer de Barretos. São Paulo, Brazil.
5 Ircad América Latina. São Paulo, Brazil.
ABSTRACT
Background: Endoscopic ultrasound training has a learning curve greater than the other endoscopic therapeutic techniques. One of the preclinical teaching methods is the use of ex vivo porcine models. Aim: To describe five ex vivo porcine models for training in therapeutic echoendoscopic procedures. Materials and methods: Using porcine digestive tract containing esophagus, stomach, duodenum, spleen, liver and gallbladder, five models for therapeutic echoendoscopy training were described. With other segments of the porcine pieces (such as the bladder, spleen segment and omentum segment) and with easily accessible materials (such as grape and ultrasound gel), lesions were simulated to be treated. These models were applied in the Hands on course at the IRCAD (Institut de recherche contre les cancers de l'appareil digestif) Barretos of 2017. Endoscopic equipment and instruments are the same as those used in clinical practice. Result: The models are easily reproducible and do not require exchange during the hands on course period. Endoscopic and echographic imaging and tactile sensitivity are similar to the real one. Conclusion: The models described in this study demonstrated to be realistic, easy to reproduce and allow repetition during the same session. However, comparative studies are necessary to verify the real impact on teaching.
Keywords: Endoscopic ultrasonography; Simulation training; Learning curve (source: MeSH NLM).
RESUMEN
Racional: El entrenamiento de la ecoendoscopía tiene una curva de aprendizaje mayor que las demás técnicas endoscópicas terapéuticas. Uno de los métodos de enseñanza preclínica es el uso de modelos porcinosex vivos. Objetivo: Describir cinco modelos porcino sex vivo para entrenamiento de procedimientos ecoendoscópicos terapéuticos. Materiales y método: Utilizando el tracto digestivo porcino, que contiene esófago, estómago, duodeno, delgado, hígado y vesícula biliar, se han descrito cinco modelos para el entrenamiento de ecoendoscopía terapéutica. Con otros segmentos de la pieza porcina (como vejiga, segmento de delgado, bazo y omento) y con materiales de fácil acceso (como uva y gel de ecografía), se simularon lesiones a ser tratadas. Estos modelos se aplicaron en el curso Handsonenel IRCAD (Institut de recherche contre les cancers de l'appareil digestif) Barretos de 2017. Los aparatos e instrumentos endoscópicos son los mismos utilizados en la práctica clínica. Resultado: Los modelos forman de fácil reproducibilidad, no siendo necesario el cambio de la pieza porcina durante el período del curso Handson. La imagen endoscópica y ecográfica y la sensibilidad táctil son similares a la real. Conclusión: Los modelos descritos en este trabajo han demostrado ser realistas, de fácil reproducción y permiten repetición durante la misma sesión. Sin embargo, los estudios comparativos son necesarios para verificar el impacto real en la enseñanza.
Palabras clave: Ecoendoscopía; Entrenamiento simulado; Curva de aprendizaje (fuente: DeCS BIREME).
INTRODUCCIÓN
Endoscopic ultrasound (EUS) is one of the most challenging endoscopic methods, since it requires knowledge of luminal endoscopy associated with echographic imaging. Because of its complexity, the learning curve requires dedication, demanding many cases executed to achieve expertise (1).
Traditionally, echoendoscopic training is performed under supervised clinical practice. According to the recommendations of the European Society of Gastrointestinal Endoscopy, ethical teaching in endoscopy is based on training with a high level of expertise, without causing harm to the patient and respecting his right to self-determination (2). Therefore there is a large investment in EUS simulators for training before clinical practice.
Currently there are five types of simulators for EUS: virtual, mechanical, phantoms, ex vivo models and use of live animals.
The use of ex vivo models has a great advantage due to the low cost, absence of ethical implications, easy reproducibility and realistic simulation of endoscopic procedures.
This article presents ex vivo models for therapeutic endoscopic ultrasound (T-EUS).
AIM
To describe five ex vivo porcine models for the training of therapeutic endoscopic ultrasound procedures.
MATERIALS AND METHODS
This study presents ex vivo models in porcine specimen consisting of the digestive tract containing esophagus, stomach, duodenum, bowel, liver, spleen and gallbladder. These pieces were cleaned, treated with formalin diluted 1%, and each model was thawed gradually 24 to 48 hours prior to the procedure, kept in cooled chambers at 8 degrees Celsius. After the preparation of each piece, the models were positioned in modified plastic models with head and torso modified for the training, as shown in the figures.
The porcine pieces were used in the hands on course at the IRCAD (Institut de recherche contre les cancers de l'appareil digestif) Barretos by the 36 participants that took place in September 2017, Barretos, São Paulo, Brazil. The training occurred in two periods of five hours each. The stations included 18 models of therapeutic endoscopy, five of them of EUS. All stations had a senior monitor supervising and guiding the procedures. All students reported having minimal training in biliopancreatic endoscopy. The equipment used was the echoendoscope GF-UCT140-AL5 and GFUCT180 with EU-ME1 processor (Olympus Optical of Brazil ltda) and monopolar electrocautery (Covidien Medtronic). The accessories used were: 0.035-inch guidewire, 19G echoendoscopic puncture needle, 10F cystotome, double pigtail biliary drainage (Wilson- Cook Medical) and self-expanding apposition metal prosthesisluminal-LAMS (M.I.Tech).
The proposed models are described below:
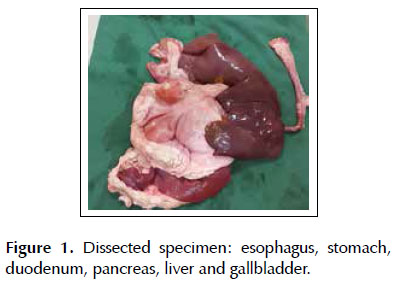
Base model: a 6 kg porcine specimen containing extracted viscera consisting of esophagus, stomach, duodenum, jejunum, liver and gallbladder positioned inside the plastic model with head and torso cut in the coronal axis. The proximal esophagus was attached to a plastic conduit tube of electrical wiring, with the aid of adjustable plastic clamps. This set was brought to the mouth of the plastic model and fixed with the clamps. The organs were positioned in the original topography. The jejunum and the diverticulum of the gastric fundus(where the gastric clearing is performed) were closed with clamps to maintain the insufflation (Figure 1).
Model 1: Study of the anatomy with EUS, diagnosis and puncture of lesions
In this model the objective was to train the basic principles of EUS, basic anatomy by echoendoscopy (echotexture of the liver, gastric wall layers), diagnosis of intramural nodules or lesions adjacent to the stomach and identification of vessels using the doppler effect.
In the porcine specimen, the lesions were implanted in several segments of the digestive tract.
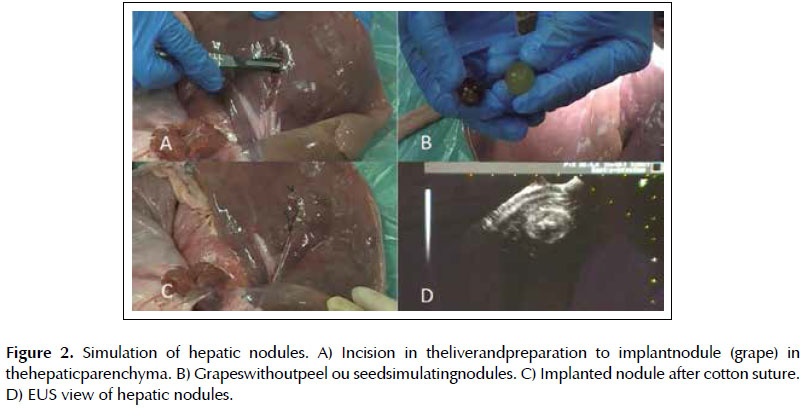
Lesion 1. Simulation of hepatic nodule. An incision was made in the hepatic parenchyma and a grape without peel or seed was placed (Figures 2A and 2B). The parenchyma was closed with 2-0 cotton suture (Figure 3C).
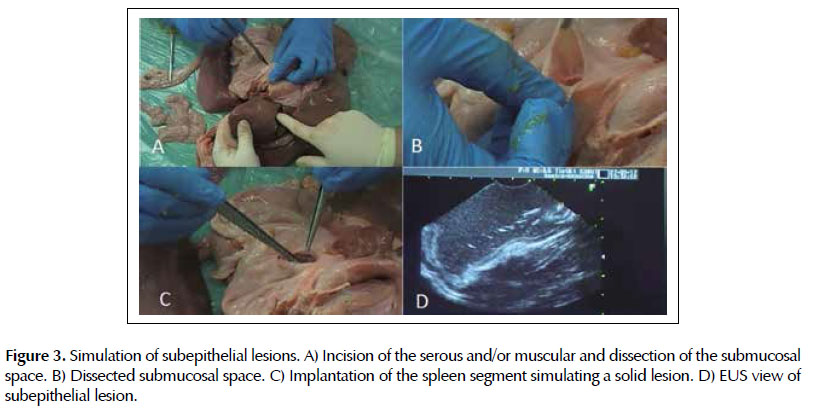
Lesion 2. Simulation of subepithelial lesions. In the esophagus or stomach the serosa and/or muscular layers was incised for insertion of fragment of omentum (lipoma) or spleen (solid lesion) on the wall, with posterior closure of the incision with 2-0 cotton suture.
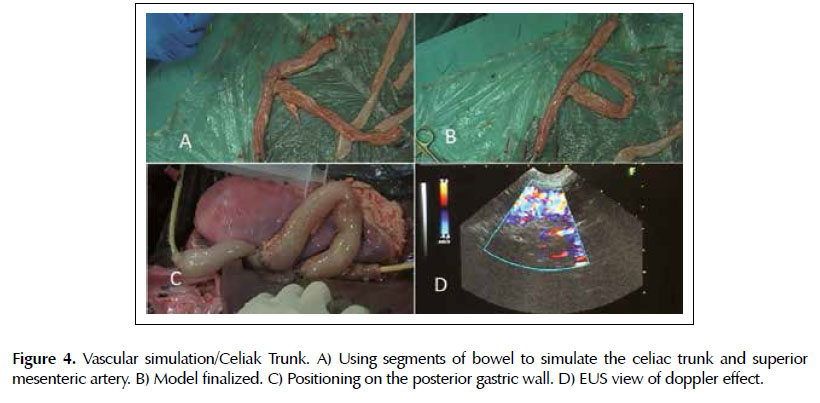
Vascular simulation. Training to localize the aorta and use the property of color doppler. A 30 cm bowel segment was filled with water and at each end were attached a Foley 20F catheter and a 60ml syringe to pass water from one end to the other, simulating blood flow (Figures 4A and 4B). This piece was then placed next to the posterior wall of the stomach and with the manipulation of the syringe plunger the color doppler effect was performed (Figures 4C and 4D).
Model 2: Fine needle aspiration by endoscopic ultrasound (FNA-EUS) of cystic lesions
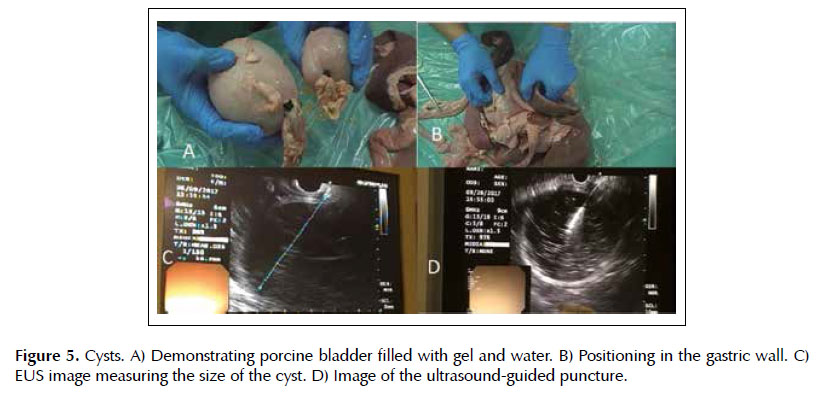
Lesion 3. Cysts. The cysts were made with porcine bladder filled by the urethra with ultrasound gel diluted in water, which was later closed with a clamp (Figure 5A). These cysts were positioned adjacent to the gastric wall at eligible sites of puncture in the base model (Figure 5B). The procedure was performed such as in clinical practice: cyst localization, use of color doppler to exclude the presence of blood vessels, puncture under ultrasound visualization, and collection of material for analysis (Figure 5D).
Model 3: Drainage of pseudocysts
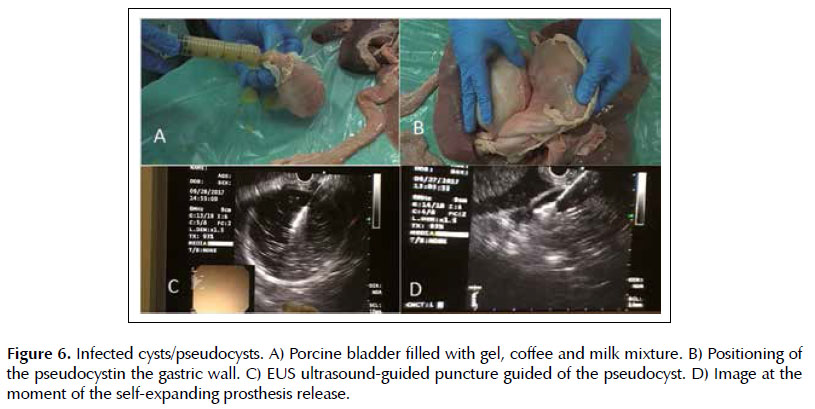
Lesion 4. Infected cysts. The pseudocysts were made with porcine bladder filled by the urethra with a mixture of ultrasound gel, milk and soluble coffee, later closed with a clamp (Figure 6A). These were positioned on the retrogastric wall. The technique is done by puncturing the pseudocyst guided by EUS, withdrawing the stylet from the needle channel and connecting the syringe for aspiration of the purulent content (Figure 6C). Subsequently removal of the syringe, passage of guidewire to maintain the path, withdrawal of the needle, passage of the 10F cystotome with electrocautery to enlarge cystogastric communication, to finally allocation of plastic or metallic prostheses. And as it is done in the patients, the release of the prostheses was monitored in real time, as shown in Figure 6D. with the passage of a self-expanding metallic apposition prostheses (endoscopic and echographic view).
Model 4: Ultrasound-guided access - choledochoduodenostomy
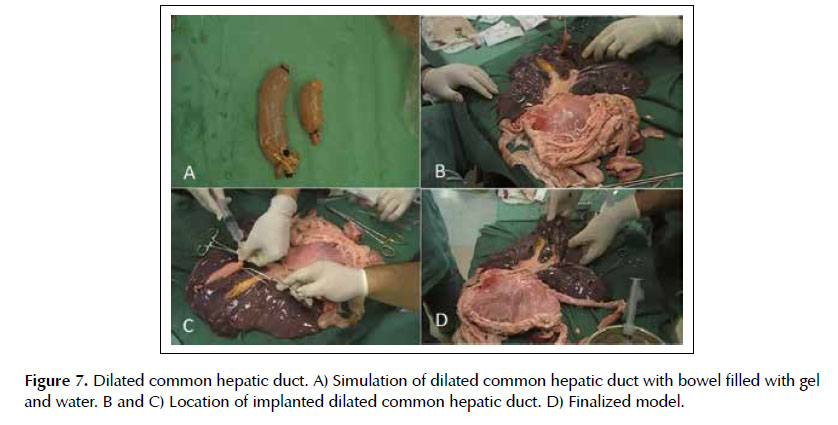
Lesion 5. Dilated common bile duct. The common bile duct was made with a 15 cm bowel segment filled with ultrasound gel diluted in water, with the ends closed with clamps (Figure 7A). This was positioned in the hepatic hilum, fixed with 2-0 cotton sutures, and its location is made by ultrasound in the duodenal bulb window (Figures 7B, 7C and 7D). After puncture of the common bile duct with 19G needle, the stylet is withdrawn from the needle channel and connection of the 20 ml syringe is made with vacuum for aspiration of the contents. Following occurs the withdrawal of the syringe, the passage of guidewire to maintain the path, the needle removal, the enlargement of the gastrocholedochal communion with the 10 F cystotome with electrocautery and the allocation of plastic or metallic prostheses.
Model 5: Ultrasound-guided access - Hepatogastrostomy
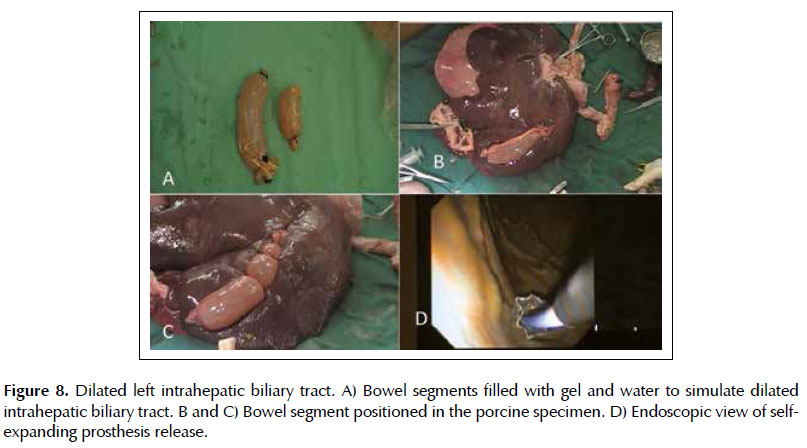
Injury 6. Dilated left intrahepatic biliary tract. The dilated intrahepatic biliary tract was made with 15 cm bowel segment filled with ultrasound gel diluted in water, with the ends closed with clamps (Figure 8A). This was positioned on the lower border of the left hepatic lobe, and fixed with 2-0 cotton sutures (Figures 8B and 8C). By ultrasound the dilated left bile duct is localized in the gastric body window. Next, it is made the puncture with a 19G needle, the withdrawn of the stylet from the needle channel and the 20ml syringe is connected with vacuum to aspirate the contents to confirm the positioning. It is performed the withdrawn of the syringe, the passage of the guidewire to maintain the path, the placement of the 10F cystotome with electrocautery to enlarge the gastrohepatic communication and, finally, the removal of the cystotome for allocation of self-expanding bile metal prostheses (Figure 8D).
RESULTS
The models demonstrated in this study proved to be durable, requiring no exchange of the porcine specimen ex vivo during the five hours of hands on course. In simple ultrasound-guided punctures, the cysts did not require many replacements and were only exchanged after complete drainage with the placement of two or more prosthesis.
The intraluminal and ultrasonographic images were similar to the human and quickly recognized. The five layers of the gastric wall were easily identified in the echographic image of this model, as well as lesions implanted in the subepithelial layers and adjacent organs (omentum segments in the gastric wall, spleen segment in the esophagus, grapes in the liver and swine bladder).
The tactile sensation was slightly more resistant, due to the greater thickness of the gastric wall and of theporcine bladder (pseudocyst), but still very similar to the procedure in humans. The movements performed with the echoendoscopy device and its positioning with the lesion are the same as those performed in clinical practice.
FNA-EUS techniques could be practiced in the same way as the real practice: diagnosis and evaluation of the lesion, positioning of the device, puncture of the nodules and withdrawal of the biopsied specimen. In addition to this, it is possible to demonstrate the ultrasound-guided puncture under external vision; in other words, in a hepatic nodule it is possible to observe in the porcine specimen the effect of the needle when crossing the gastric wall. Situation that does not occur in the anesthetized live model. The same similarity in the technique was found in drainage.
The ultrasound access mimicking the choledochoduodenostomy and hepatogastrostomy was shown to be highly didactic and the hands on participants demonstrated success in their entirety.
All the steps of the endoscopic procedures, such as the exchange of guidewire, passage of metallic prosthesis, visualization of the needle in EUS and the release of prosthesis were identical, since we used the same equipment and endoscopic instruments of clinical practice.
DISCUSSION
In a review of the guideline of the European Society of Gastrointestinal Endoscopy it is reported that 8 to 50% of the echoendoscopists have informal training and are usually self-taught (3). This situation is discouraged by the European Society and more and more training directly on patients will be less accepted.
The American Society of Gastrointestinal Endoscopy formulated a guideline to establish the minimum amount of EUS training to achieve proficiency. It recommends at least 150 echoendoscopic procedures, including 75 cases of pancreas and biliary tract and 40 cases of submucosal lesions. And at least 50 cases of FNA-EUS are required, 25 of them with pancreatic lesions (4).
The large proportion of self-taught physicians is due to limited access to EUS centers (5). And even for those receiving formal training, there is not necessarily enough amount to achieve the proficiency required by the guideline. A survey conducted with directors of fellowship programs with EUS training revealed that the number of echoendoscopic procedures performed by the fellows is low, with a median of 50. About 60% of the programs do not allow a fellow to be performed a FNA-EUS in the third year of training. More than 40% of the fellows learn EUS by theoretical and observational methods, without hands on access. It was concluded in this research that even formal training is currently insufficient compared to the guideline recommendations of the American Society (6).
Therefore, the role of simulators for EUS training becomes important. Not to replace clinical training, but to reduce the learning curve.
In some areas of endoscopy, the effectiveness of simulator training before clinical practice has already been proven. In the study by Cohen et al of colonoscopic training, the group that used the virtual simulator for eight weeks had significantly better performance than the control group (7). Martinek et al made a randomized study to evaluate hemostasis and perforation treatment with upper gastrointestinal endoscopy in ex vivo models in a two-day training. In this study, physicians who underwent training had an improvement in the technique of clipping, coagulation and injection (8). The same result was found in the endoscopic hemostasis study of Hochberger et al with evaluation of a group with clinical standard training against a group that performed training with the ex vivo model associated (9).
Even for experienced echoendoscopists, simulators can be used during the application of new techniques and new endoscopic instruments, since the practice directly in the patients in these situations is related to high rates of complications and surrounded by ethical issues (10).
The simulators currently available for EUS training are: phantoms, virtual simulators, mechanical simulators, live animals and ex vivo models.
The first one cited is a model with the intention of practicing FNA-EUS. It consists of a box filled with silicone or agar in which targets are implanted (blocks, grapes, vegetables) to mimic the puncture of cysts or nodules. It is a cheap and portable method, and can be useful in the beginning of learning with the echoendoscope. However not at all realistic, not simulating anatomy or real life conditions (5,11).
The first virtual simulators were developed in the 80's. These have demonstrated a satisfactory visual reality, allowing several situations to be simulated and repeated in a controlled environment and with feedback during the realization of the cases. However, they lack tactile reality and their costs are still high (5,12,13). Virtual simulators have a proven impact when used in the initial stages of the training of new endoscopists in gastroscopy and colonoscopy (14).
There are also mechanical simulators, which try to reproduce the human anatomy using materials such as plastic tubes, rubbers, foam and acrylics. However, these models are commonly used for endoscopic retrograde cholangiopancreatography and there was little development for the EUS model (15).
The most realistic model we currently have is the use of anesthetized live animals due to tactile sensitivity, presence of blood flow, and especially human-like anatomy: mediastinum, pancreas, bile duct, spleen, gastrointestinal wall, and vessels of the gastrointestinal tract (celiac trunk, mesenteric vessel, portal vein, and so) (10,16). However, they are difficult to acquire, expensive, need a larger structure and, depending on the country, barring ethical and legal principles (5).
Moreover, it does not allow to simulate many pathologies and certain situations cannot be recreated for further training after the first procedure, such as a gallbladder puncture.
Yet the exvivo model is based on the use of porcine pieces. The first report of swine stomach for gastroscopy occurred in 1995. The following year, Hochberger and Neumann developed the EASIE (Erlanger Ausbildungssimulator für die Interventionelle Endoskopie), or the Erlanger Interventional Endoscopic Training Simulator. Since then, this model has been improved to train endoscopists (17).
In this model it is possible to simulate EUS with a linear or radial device, it has low cost, allows the use of real endoscopic accessories, without time limitation, in a controlled environment, being able to repeat the procedure countless times (5,11,18). But the greatest advantages are the tactile sensation and a human-like anatomy.
It was Buthani and cols, in 1998, who described the use of an ex vivo porcine model for EUS training, reporting the similarity to the human anatomy in the five layers of the gastric wall, in the echotexture of the liver, pancreas, and spleen (19).
Using an ex vivo model, Gonzalez and cols performed one of the few prospective studies for EUS training. After each fellow performed 30 FNA-EUS, there was an improvement in time, number of puncture attempts, scores on device handling skills and reduction in time in which the needle was lost to EUS (20).
In order to improve this method of endoscopic teaching, in the present study several models for T-EUStraining were recreated. Allowing evaluation of the basic anatomy, drainage of collections and pseudocysts, punctures of cysts, transmural lesions and liver lesions.
It should be emphasized that the different simulators are not excludents but complementary. According to Kim et al, the passive teaching method with books, videos and media is first suggested; secondarily virtual simulators for notions of device handling and anatomy; followed by the use of phanthoms for notions of punctures; and finally the ex vivo models or live animals that are at the plateau of the most realistic methods. They also reaffirm that simulators improve training, but do not replace supervised clinical training (5).
CONCLUSION
Five ex vivo models have been described for the training of T-EUSprocedures. The models are easy to reproduce, demonstrate full degree of realism and allow repetition during the same session. However, comparative studies between these models and procedures in humans are necessary to verify the real impact on teaching.
BIBLIOGRAPHIC REFERENCES
1. Goodman AJ, Gress FG. Advances in simulation of diagnostic and therapeutic endoscopic ultrasound. Tech Gastrointest Endosc. 2011;13(3):183-7. [ Links ]
2. Axon AT, Aabakken L, Malfertheiner P, Danielides I, Ladas S, Hochberger J, et al. Recommendations of the ESGE Workshop on ethics in teaching and learning endoscopy. Endoscopy. 2003;35(9):761-4. [ Links ]
3. Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44(2):190-205. [ Links ]
4. Eisen GM, Dominitz JA, Faigel DO, Goldstein JA, Petersen BT, Raddawi HM, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastroint Endosc. 2001;54(6):811-4. [ Links ]
5. Kim GH, Bang SJ, Hwang JH. Learning models for endoscopic ultrasonography in gastrointestinal endoscopy. World J Gastroenterol. 2015;21(17):5176-82. [ Links ]
6. Azad JS, Verma D, Kapadia AS, Adler DG. Can U.S. GI fellowship programs meet American Society for Gastrointestinal Endoscopy recommendations for training in EUS? A survey of U.S. GI fellowship program directors. Gastrointest Endosc. 2006;64(2):235-41. [ Links ]
7. Cohen J, Cohen SA, Vora KC, Xue X, Burdick JB, Bank S, et al. Multicenter, randomized, controlled trial of virtualreality simulator training in acquisition of competency in colonoscopy. Gastrointest Endosc. 2006;64(3):361-8. [ Links ]
8. Martinek J, Suchanek S, Stefanova M, Rotnaglova B, Zavada F, Strosova A, et al. Training on an ex vivo animal model improves endoscopic skills: a randomized, single-blind study. Gastrointest Endosc. 2011;74(2):367-73. [ Links ]
9. Hochberger J, Matthes K, Maiss J, Koebnick C, Hahn EG, Cohen J. Training with the compactEASIE biologic endoscopy simulator significantly improves hemostatic technical skill of gastroenterology fellows: a randomized controlled comparison with clinical endoscopy training alone. Gastrointest Endosc. 2005;61(2):204-15. [ Links ]
10. Parra-Blanco A, González N, González R, Ortiz-Fernández- Sordo J, Ordieres C. Animal models for endoscopic training: do we really need them? Endoscopy. 2013;45(6):478-84. [ Links ]
11. Gromski MA, Matthes K. Simulation in advanced endoscopy: state of the art and the next generation. Tech Gastrointest Endosc. 2011;13(3):203-8. [ Links ]
12. Baron TH, DeSimio TM. New ex-vivo porcine model for endoscopic ultrasoundguided training in transmural puncture and drainage of pancreatic cysts and fluid collections. Endosc Ultrasound. 2015;4(1):34-9. [ Links ]
13. Matsuda K, Tajiri H, Hawes RH. How shall we esperience EUS and EUS-FNA before the first procedure? The development of learning tools. Dig Endosc. 2004;16:S236-9. [ Links ]
14. Triantafyllou K, Lazaridis LD, Dimitriadis GD. Virtual reality simulators for gastrointestinal endoscopy Training. World J Gastrointest Endosc. 2014;6(1):6-12. [ Links ]
15. Pawa R, Chuttani R. Benefits and limitations of simulation in endoscopic training. Tech Gastrointest Endosc. 2011;13(3):191-8. [ Links ]
16. Fernandez-Sordo JO, Madrigal-Hoyos E, Waxman I. The role of live animal models for teaching endoscopy. Tech Gastrointest Endosc. 2011;13(2):113-8. [ Links ]
17. Maiss J, Naegel A, Hochberger J. The European experience— current use of simulator training in Europe. Tech Gastrointest Endosc. 2011;13(2):126-31. [ Links ]
18. Matthes K, Thakkar SJ. See one, simulate one, do one, teach one—the value of hands-on simulator training in interventional endoscopy. Tech Gastrointest Endosc. 2011;13(2):111-2. [ Links ]
19. Bhutani MS, Aveyard M, Stills HF Jr. Improved model for teaching interventional EUS. Gastrointest Endosc. 2000;52(3):400-3. [ Links ]
20. Gonzalez JM, Cohen J, Gromski MA, Saito K, Loundou A, Matthes K. Learning curve for endoscopic ultrasound-guided fineneedle aspiration (EUS-FNA) of pancreatic lesions in a novel ex-vivo simulation model. Endosc Internat Open. 2016;4(12):E1286-91. [ Links ]
Correspondencia:
Everson L. A. Artifon
Rua Guimaraes Passos, 260/apto 121 Sao Paulo. ZC: 04107-030; Brazil
E-mail: eartifon@hotmail.com
Recibido: 28-11-2017
Aporbado: 14-2-2018