Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Gastroenterología del Perú
Print version ISSN 1022-5129
Rev. gastroenterol. Perú vol.38 no.2 Lima Apr./Jun. 2018
ARTÍCULOS ORIGINALES
Percutaneous endoscopic gastrostomy in children: a single center study at Tertiary hospital Iran
Gastrostomía percutánea en niños: reporte de un hospital terciario de Iran
Seyed Mohsen Dehghani1, Mahmood Haghighat1, Farideh Nematollahi1, Hazhir Javaherizadeh2,3, Naser Honar1, Maryam Bahmanyar4, Maryam Ataollahi1
1 Gastroenterohepatology Research Center, Shiraz University of Medical Sciences. Shiraz, Iran.
2 Depar tment of Pediatric Gastroenterology, Abuzar Childrens' Hospital, Ahvaz Jundishapur University of Medical Sciences. Ahvaz, Iran.
3 Alimentary Tract Research Center, Ahvaz Jundishapur University of Medical Sciences. Ahvaz, Iran.
4 Depar tment of Pediatrics, Valiasr Hospital, Fasa School of Medicine. Fasa, Iran.
ABSTRACT
Introduction: The aim of this study was to evaluate complications after percutaneous endoscopic gastrostomy among children who underwent percutaneous endoscopic gastrostomy in Nemazee hospital. Materials and methods: All children who underwent percutaneous endoscopic gastrostomy were included in the current study. Place of the study was department of pediatric gastroenterology of Nemazee children hospital of Shiraz university of medical sciences. Duration of the study was 5 year starting from 2008. All drugs such as aspirin, NSAIDS, and heparin were discontinued 1-7 days before procedures. All patients were kept NPO 6-8 hours before procedure according to the age. Single dose antibiotic was prescribed for all cases before procedure. During procedure, all patients were sedated using propofol and or midazolam. Some patients required intubation. Results: Of 39 cases who underwent PEG, 4 (10.2%) patients showed complication. The most common indication for PEG insertion were neurologic problem (84.6%) and metabolic disease (10.2%). Of our patients, 84.6% of the cases had the weight below third percentile. Conclusion: The most common indication for percutaneous endoscopic gastrostomy was cerebral palsy. The complication rate in our study was 10.2%. Celulitis was the most common complication.
Keywords: Endoscopy; Gastrostomy; Child (source: MeSH NLM).
RESUMEN
Introducción: El objetivo de este estudio fue evaluar las complicaciones luego de una gastrostomía endoscópica percutánea (PEG) en niños realizada en el hospital Nemazee. Material y métodos: Se incluyeron al estudio todos los niños que se realizaron PEG en el hospital. El lugar del estudio fue el departamento de gastroenterología pediátrica del Hospital para niños Nemazee de la Universidad Shiraz de ciencias médicas. La duración del estudio fue cinco años, iniciando en el año 2008. Todas las drogas como aspirina, AINES y heparina fueron suspendidas entre 1 a 7 días previos al procedimiento. Todos los pacientes estuvieron entre 6 a 8 horas del examen en ayunas dependiendo de la edad. Se prescribió una dosis de antibioterapia profiláctica en todos los casos previo al procedimiento. Durante el procedimiento, todos los pacientes fueron sedados usando propofol y/o midazolam. Algunos pacientes necesitaron intubación. Resultados: De 39 casos que se sometieron a PEG, 4 (10,2%) tuvieron alguna complicación. La indicación más frecuente de PEG fueron los problemas neurológicos (84,6%) y luego las enfermedades metabólicas (10,2%). De nuestros pacientes, 84,6% de los casos estuvieron por debajo del tercer percentile. Conclusión: La indicación más común de gastrostomía endoscópica percutánea fue la parálisis cerebral. La tasa de complicación en nuestro estudio fue 10,2%. La celulitis fue la complicación más común.
Palabras clave: Endoscopía; Gastrostomía; Niño (fuente: DeCS BIREME).
INTRODUCTION
Adequate nutrition is mandatory for growth and development for every child. In some situation, alternative routes for feeding is recommended. Nasogastric tube is one of the first option for feeding of children. In children with prolonged nasogastric tube feeding lasting more than 3 months, percutaneous endoscopic gastrostomy is recommended. Percutaneous endoscopic gastrostomy was first described by Gauderer at 1980 (1). The first percutaneous gastrostomy was done in a 4.5 months infant (1).
There was no report of Iran about percutaneous endoscopic gastrostomy in children according to Pubmed result.
The aim of this study was to evaluate complication of percutaneous endoscopic gastrostomy in children.
MATERIALS AND METHOD
All children who underwent percutaneous endoscopic gastrostomy were included in the current study. Place of the study was department of pediatric gastroenterology of Nemazee children hospital of Shiraz university of medical sciences. Duration of the study was 5 year starting from 2008.
All drugs such as aspirin, NSAIDS, and heparin were discontinued 1-7 days before procedures. All patients were kept NPO 6-8 hours before procedure according to the age. Single dose antibiotic was prescribed for all cases before procedure. During procedure, all patients were sedated using propofol and or midazolam. Some patients required intubation.
Early complications were evaluated during the first week after procedure. Late complications were evaluated after 1 week. All subjects with coagulopathy (INR>1.5, PTT>50 second), thrombocytopenia (<50*109/L), severe ascites, peritonitis, esophageal obstruction, or hemodynamically unstable were not good candidate for PEG.
RESULTS
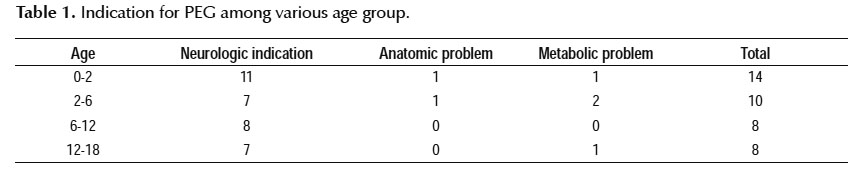
During study period, 45 percutaneous endoscopic gastrostomy was done. Three were excluded due to unavailable information. Three were reinsertion of PEG. Finally, 39 cases were included in our study. Of 39 cases, 21 (53.8%) were male and 18 (46.2) were female. Indication of PEG was shown in Table 1.
Neurological problem (n=33) were the most common indication (Table 1).
Of 39 cases who underwent PEG, 4 (10.2%) patients showed complication. Two children showed local infection. Infection resolved with oral and systemic antibiotics. One case showed displaced gastrostomy tube. Abdominal distention and leakage was occurred in one case. PEG was removed for this case.
DISCUSSION
In children with prolonged nasogastric tube feeding lasting more than 3 months, percutaneous endoscopic gastrostomy is recommended.
The most common indication of PEG in our study was cerebral palsy. In the study by Sathesh-Kumar et al. (2), the most common indication was cerebral palsy and was similar to our study.
Among all cases, complications were seen in 10.2% of the cases. In the study from Park et al. (3) on 32 children with neurological problem whom underwent PEG, complications were reported in 47% of the cases which was significantly higher than our study. In the study by Liu et al., early and late complication rates were 10.5% and 41.9%, overall complication rate was 54.7% following PEG (4). In another study, 10.5% of cases experience at least one complication (5). In the study by Ségal et al. (6), among 110 children with PEG insertion with follow up duration between 1 to 8 years, the overall rate of late onset complication was 44%. Follow up period in our study was shorter than Ségal et al. study (6).
In the current study, two children of 39 cases had local infection. Cellulitis at the gastrostomy wound were reported in 14 of 83 pediatric patients by Wu et al. (7), which was higher than our study. In another study, of 26 children with PEG insertion, three patients had immediate post-operative complication: two had leakage and one had infection (8). Infection rate in our study was similar to Bergmeijer et al. study (8).
Gastrocolic fistula was not reported among our cases. In the study by Landisch et al., gastrocolic fistula was seen in 3.8% of the infants underwent PEG insertion (9).
There was no report of death following gastrostomy in our institution. This was similar to the report from South Africa by van der Merve et al. (10). In the study by Lalanne et al., in 368 children between 1990-2003, two death was reported following PEG (11).
In conclusion, as seen above the rate of complication following percutaneous endoscopic gastrostomy in our study was similar or lower than most of the studies.
ACKNOWLEDGMENT
This study was supported by research affair of Shiraz University of Medical Sciences (No#92-01-21-6231).
Limitation: Duration of follow up is the major limitation of this study.
Conflict of interest: Nothing.
BIBLIOGRAPHIC REFERENCES
1. Gauderer MW, Ponsky JL, Izant RJ, Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872-5. [ Links ]
2. Sathesh-Kumar T, Rollins H, Cheslyn-Curtis S. General paediatric surgical provision of percutaneous endoscopic gastrostomy in a district general hospital–a 12-year experience. Ann R Coll Surg Engl. 2009;91(5):404-9. [ Links ]
3. Park JH, Rhie S, Jeong SJ. Percutaneous endoscopic gastrostomy in children. Korean J Pediatr. 2011;54(1):17-21. [ Links ]
4. Liu R, Jiwane A, Varjavandi A, Kennedy A, Henry G, Dilley A, et al. Comparison of percutaneous endoscopic, laparoscopic and open gastrostomy insertion in children. Pediatr Surg Int. 2013;29(6):613-21. [ Links ]
5. McSweeney ME, Kerr J, Jiang H, Lightdale JR. Risk factors for complications in infants and children with percutaneous endoscopic gastrostomy tubes. J Pediatr. 2015;166(6):1514-9.e1. [ Links ]
6. Segal D, Michaud L, Guimber D, Ganga-Zandzou PS, Turck D, Gottrand F. Late-onset complications of percutaneous endoscopic gastrostomy in children. J Pediatr Gastroenterol Nutr. 2001;33(4):495-500. [ Links ]
7. Wu FY, Wu JF, Ni YH. Long-term outcome after percutaneous endoscopic gastrostomy in children. Pediatr Neonatol. 2013;54(5):326-9. [ Links ]
8. Bergmeijer JH, Postuma MC, Madern GC, Bouquet J. [Favorable results of percutaneous endoscopic gastrostomy in children in need of long-term tube feeding]. Ned Tijdschr Geneeskd. 1997;141(5):241-3. [ Links ]
9. Landisch RM, Colwell RC, Densmore JC. Infant gastrostomy outcomes: The cost of complications. J Pediatr Surg. 2016;51(12):1976-82. [ Links ]
10. van der Merwe WG, Brown RA, Ireland JD, Goddard E. Percutaneous endoscopic gastrostomy in children--a 5-year experience. S Afr Med J. 2003;93(10):781-5. [ Links ]
11. Lalanne A, Gottrand F, Salleron J, Puybasset-Jonquez AL, Guimber D, Turck D, et al. Long-term outcome of children receiving percutaneous endoscopic gastrostomy feeding. J Pediatr Gastroenterol Nutr. 2014;59(2):172-6. [ Links ]
Correspondence:
Hazhir Javaherizadeh
Alimentary Tract Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
E-mail: hazhirja@yahoo.com, hazhirja@ajums.ac.ir
Recibido: 06-09-2017
Aprobado: 12-02-2018