Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Gastroenterología del Perú
Print version ISSN 1022-5129
Rev. gastroenterol. Perú vol.38 no.2 Lima Apr./Jun. 2018
ARTÍCULOS ORIGINALES
Effect of sequential therapy on treatment of Helicobacter pylori infection in children
Efecto de la terapia secuencial en el tratamiento de la infección por Helicobacter pylori en niños
Seyed Mohsen Dehghani1, Afsaneh Nazari2, Hazhir Javaherizadeh3
1 Gastroenterohepatology Research Center, Nemazee Teaching Hospital, Shiraz University of Medical Sciences. Shiraz, Iran.
2 Department of Pediatrics, Nemazee Teaching Hospital, Shiraz University of Medical Sciences. Shiraz, Iran.
3 Alimenatry Tract Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
ABSTRACT
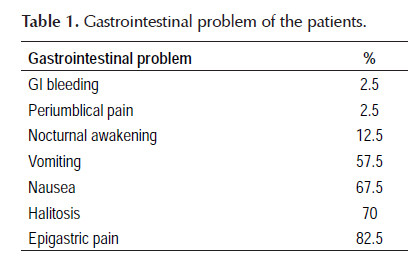
Background: Helicobacter pylori infection which plays a major role in the etiology of chronic gastritis and duodenal ulcers in children and adults is one of the commonest chronic infection worldwide. Cure of the infection leads to healing of gastric inflammation and prevention of peptic ulcer. Objective: The aim of this study was to evaluate the efficacy of the sequential therapy for treatment of Helicobacter pylori infection. Materials and methods: In this study, 40 children with symptoms of H. Pylori that the infection was proved by endoscopy and biopsy and rapid urease test (UBT) were enrolled, and received sequential therapy (Lansoprazol, Amoxicillin) for 5 days and (Lansoprazol, Metronidazole and Clarithromycin) for next 5 days. The eradication rate of therapy was evaluated by stool antigen test 6 weeks after completion of therapy. This study was carried out in Pediatric Gastroenterology Clinic of Shiraz University of Medical Sciences, Shiraz, Iran. This study was approved by ethic committee of Shiraz University of Medical Sciences. Results: Forty children with mean age of (10.8±4 years) were evaluated. The most common symptom on first admission was epigastric pain (82.5%), with mean duration of symptoms (16±14.5 month). The most common endoscopic findings was redness and erosion of the antrum (55%) and the most pathologic findings was chronic gastritis (77.5%). The most drug adverse effect was nausea (22.5%). The eradication rate of sequential therapy was 82.5%. Conclusion: Eradication rate of sequential therapy was 82.5% among our cases.
Keywords: Helicobacter pylori; Terapéutica; Eradication (source: MeSH NLM).
RESUMEN
Antecedentes: La infección por Helicobacter pylori, que juega un rol principal en la etiología de la gastritis crónica y las úlceras duodenales en niños y adultos, es una de las infecciones crónicas más comunes en el mundo. La cura de esta infección lleva a la cura de la inflamación gástrica y a la prevención de la úlcera péptica. Objetivo: Evaluar la eficacia de la terapia secuencial en el tratamiento de la infección por Helicobacter pylori. Material y métodos: En este estudio, se enrolaron 40 niños con síntomas en los que la infección por H. pylori se demostró por endocopía con biopsia y prueba rápida de ureasa (UBT) y recibieron terapia secuencial (Lansoprazol, Amoxicilina) por 5 días y (lansoprazol, metronidazol y clarotromicina) por otros 5 días. La tasa de erradicación de la terapia se evaluó por prueba de antígeno en heces 6 semanas después de terminar la terapia. Este estudio se llevó a cabo en la Clínica de Gastroenterología Pediátrica de la Universidad de Ciencias Médicas de Shiraz, Irán. El estudio fue aprobado por el comité de ética de la Universidad de Ciencias Médicas de Shiraz. Resultados: Se evaluaron cuarenta niños con una edad media de (10,8±4 años). El síntoma más común al ingreso fue dolor epigástrico (82,5%) con una duración media de síntomas de (16±14,5 meses). El hallazgo endoscópico más común fue enrojecimiento y erosión del antro (55%) y el hallazgo patológico más común fue gastritis crónica (77,5%). El evento adverso más común fue náusea (22,5%). La tasa de erradicación de la terapia secuencial fue 82,5%. Conclusión: La tasa de erradicación de la terapia secuencial fue de 82,5% en nuestros casos.
Palabras clave: Helicobacter pylori; Therapeutics; Erradicación (fuente: DeCS BIREME).
INTRODUCTION
Helicobacter pylori is a S-shaped gram negative bacilli that produces urease, catalase, and oxidase. Some treatment strategy that was appropriate in adults may not applicable in children (1,2).
Initial studies of sequential therapy suggested that its superiority over standard triple therapy might be due to improved eradication of clarithromycin resistant strains (3).
First line of treatment consists of triple drugs that contains proton pump inhibitor and two antibiotics of metronidazole, clarithromycin, or amoxicillin for 7 to 14 days (4). The sequential therapy consists of a PPI and amoxicillin for the first 5 days followed by a PPI and two other antibiotics for the next five days (3).
The aim of this study was to evaluate Helicobacter pylori eradication rate following sequential therapy.
MATERIALS AND METHODS
Forty children aged <18 years with gastrointestinal symptoms with suspected H. pylori infection were included. Duration of study was 1 year. Patients with abdominal pain, nausea, vomiting, halitosis, and gastrointestinal bleeding were undergoing upper gastrointestinal endoscopy. Rapid urease test and histopathology evaluation were done for cases. Subjects with evidence of H. pylori infection according to histopathology examination and rapid urease test were included in our study. Sequential therapy was started for all eligible patients. Sequential therapy includes amoxicillin 50 mg/kg/day, lansoprazole 1 mg/ kg/day for the first 5 days. Clarithromycin 20 mg/kg/ day, metronidazole 20 mg/kg/day, and lansoprazole 1 mg/kg/day for 5 days were prescribed for next 5 days. Demographic features were recorded.
Patients were followed for adverse effect of treatment such as nausea, abdominal cramps, diarrhea, and skin rash.
Helicobacter pylori eradication was assessed using stool antigen test which was performed 6 weeks after treatment. Negative stool antigen was considered as a successful treatment. H. Pylori antigen detection in stool is a rapid, non-invasive, and easy to perform test that can be used to detect infection and monitoring after treatment (5,6).
Data was analyzed using SPSS version 19.0. p value <0.05 was considered significant.
This study was approved by ethical committee of the university (Ethic code: IR.SUMS.MED.REC.1394.54. This clinical trial study was registered in Iranian Registry of Clinical Trial numbered IRCT201702179101N3. Informed consents was signed by parents and caregivers.
RESULTS
In this study 40 (m=23, f=17) children were studied. Mean age of the cases was 10.8±4 years old. Primary symptoms of the cases were shown in Table 1.
After 6 weeks, children were evaluated in terms of their symptoms. Symptoms were resolved in 24 (60%) of the cases completely. Sixteen (40%) patients remained symptomatic.
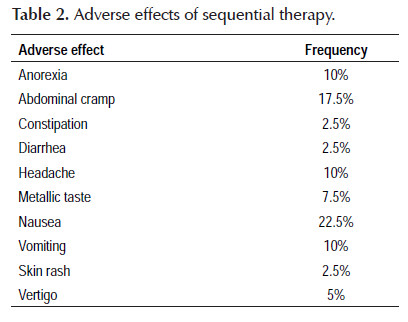
Compliance of the treatment was acceptable in 90% of cases. Drug adverse effect was seen in 60% of cases. Nausea was noted in 22.5% of cases. Abdominal cramps was seen in 17.5% of cases. Anorexia was seen in all cases, 45% of patients had positive family history of gastrointestinal disease. Table 2.
Endoscopic findings before treatment are redness and ulcer in antrum (55%), nodularity in antrum (30%), esophagitis with mucosal redness of stomach (10%), and simultaneous gastric and duodenal ulcer (5%).
DISCUSSION
Redness and ulcer in antrum area were the most endoscopic findings among our cases. In the study by Bujanover et al., antral redness and mucosal nodularity were the most findings in patients with helicobacter pylori infection (7).
Nausea and vomiting were the most frequent adverse effect among patients. In another study by Kutluk et al., abdominal pain and abdominal cramps were the most frequent complication (8). In the current study, nauseas was noted in 22.5% of the cases. In Dehghani et al. study, nausea was less frequent among children who treated with other regimes (9).
Headache was found in 10% of the cases. In the previous study by Dehghani et al., 30.8% of the patients treated by omeprazole-amoxicllin-bismuth had headache that was significantly higher than sequential therapy. Headache was more frequent in children treated by (omeprazole, amoxicillin, metronidazole, and bismuth subcitrate) or (omeprazole, amoxicillinclavulanic acid, and metronidazole) than the current study (9).
Abdominal cramp was found in 17.5% of children in our study. The frequency of abdominal cramp was lower than other regimens that studied in Dehghani et al. published report that was seen in 19.5% to 35.1% of children (9).
In the current study, metallic test was reported by 7.5% of the cases. In Dehghani et al. study, metallic taste was reported by 7.7%-51.3% cases regarding to different regimens (9).
Skin rash was reported by 2.5% of the cases which is similar to the previous study from Shiraz-Iran (9).
Eradication rate was 82.5% in our study. Eradication rate was 84.6% in Laving et al. study (10). In Huang et al. (11) and Gatta et al. (12) study, eradication rate was 81.4% and 84.3% respectively. Liu KS reported 89% eradication rate for sequential therapy (13). In another study from Poland, Helicobacter pylori eradication rate was 90.48% using sequential therapy (14).
In Francavilla and colleagues study, 36 and 42 patients received sequential therapy and classic triple therapy (15). Eradication rate was higher in sequential therapy compared to triple therapy (15). In Dehghani et al. study, eradication rate by quadruple therapy with omeprazole; amoxicillin; metronidazole; and bismuth had eradication rate about 91.9% that was higher than sequential therapy (9). In a recent study on adult patients, eradication rate was higher in sequential therapy compared triple therapy (16). In contrast, in another study eradication rate was similar between sequential therapy and conventional therapy (17).
In our study, half of the cases had history of peptic disease among first degree relatives which is similar to Dehghani et al. study (9).
There is some difference between studies in term of method of assessment of eradication. In the current study, negative stool antigen was used. In the previous study by Dehghani et al., urea breath test was used (9). In the recent study by Honar et al., they showed that sensitivity and specificity of UBT in detection of H. pylori infection was lower than expected (18). So, some results may vary according to the method of post treatment evaluation.
Most of the studies that published about treatment of H. pylori were from adult population. Further well designed studies are needed in children.
ACKNOWLEDGMENT
This study was supported by Shiraz University of Medical Sciences (Reg no=92-01-01-6935).
Conflict of interest: None
Source of funding: Shiraz University of Medical Sciences
Study limitation: Low sample size and single center study.
BIBLIOGRAPHIC REFERENCES
1. Wewer V, Kalach N. Helicobacter pylori infection in pediatrics. Helicobacter. 2003;8 Suppl 1:61-7. [ Links ]
2. Veres G, Pehlivanoglu E. Helicobacter pylori infection in pediatrics. Helicobacter. 2007;12 Suppl 1:38-44. [ Links ]
3. Zullo A, Vaira D, Vakil N, Hassan C, Gatta L, Ricci C, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther. 2003;17(5):719-26. [ Links ]
4. O'Connor A, Gisbert JP, O'Morain C, Ladas S. Treatment of Helicobacter pylori Infection 2015. Helicobacter. 2015; 20 Suppl 1:54-61. [ Links ]
5. Martin JJ, Anbumani N, Kalyani M, Rajesh PK. H. pylori antigen detection in stool. Indian J Med Microbiol. 2006;24(1):79-80. [ Links ]
6. Konstantopoulos N, Russmann H, Tasch C, Sauerwald T, Demmelmair H, Autenrieth I, et al. Evaluation of the Helicobacter pylori stool antigen test (HpSA) for detection of Helicobacter pylori infection in children. Am J Gastroenterol. 2001;96(3):677-83. [ Links ]
7. Bujanover Y, Konikoff F, Baratz M. Nodular gastritis and Helicobacter pylori. J Pediatr Gastroenterol Nutr. 1990;11(1):41-4. [ Links ]
8. Kutluk G, Tutar E, Bayrak A, Volkan B, Akyon Y, Celikel C, et al. Sequential therapy versus standard triple therapy for Helicobacter pylori eradication in children: any advantage in clarithromycin-resistant strains? Eur J Gastroenterol Hepatol. 2014;26(11):1202-8. [ Links ]
9. Dehghani SM, Erjaee A, Imanieh MH, Haghighat M. Efficacy of the standard quadruple therapy versus triple therapies containing proton pump inhibitor plus amoxicillin and clarithromycin or amoxicillin-clavulanic acid and metronidazole for Helicobacter pylori eradication in children. Dig Dis Sci. 2009;54(8):1720-4. [ Links ]
10. Laving A, Kamenwa R, Sayed S, Kimang'a AN, Revathi G. Effectiveness of sequential v. standard triple therapy for treatment of Helicobacter pylori infection in children in Nairobi, Kenya. S Afr Med J. 2013;103(12):921-4. [ Links ]
11. Huang J, Zhou L, Geng L, Yang M, Xu XW, Ding ZL, et al. Randomised controlled trial: sequential vs. standard triple therapy for Helicobacter pylori infection in Chinese children-a multicentre, open-labelled study. Aliment Pharmacol Ther. 2013;38(10):1230-5. [ Links ]
12. Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. [ Links ]
13. Liu KS, Hung IF, Seto WW, Tong T, Hsu AS, Lam FY, et al. Ten day sequential versus 10 day modified bismuth quadruple therapy as empirical firstline and secondline treatment for Helicobacter pylori in Chinese patients: an open label, randomised, crossover trial. Gut. 2014;63(9):1410-5. [ Links ]
14. Iwanczak BM, Borys-Iwanicka A, Biernat M, Go ciniak G. Assessment of Sequential and Standard Triple Therapy in Treatment of Helicobacter pylori Infection in Children Dependent on Bacteria Sensitivity to Antibiotics. Adv Clin Exp Med. 2016;25(4):701-8. [ Links ]
15. Francavilla R, Lionetti E, Castellaneta SP, Magistà AM, Boscarelli G, Piscitelli D, et al. Improved efficacy of 10-Day sequential treatment for Helicobacter pylori eradication in children: a randomized trial. Gastroenterology. 2005;129(5):1414-9. [ Links ]
16. Chung JW, Han JP, Kim KO, Kim SY, Hong SJ, Kim TH, et al. Ten-day empirical sequential or concomitant therapy is more effective than triple therapy for Helicobacter pylori eradication: A multicenter, prospective study. Dig Liver Dis. 2016;48(8):888-92. [ Links ]
17. Das R, Sureshkumar S, Sreenath GS, Kate V. Sequential versus concomitant therapy for eradication of Helicobacter pylori in patients with perforated duodenal ulcer: A randomized trial. Saudi J Gastroenterol. 2016;22(4):309-15. [ Links ]
18. Honar N, Minazadeh A, Shakibazad N, Haghighat M, Saki F, Javaherizadeh H. Diagnostic accuracy of urea breath test for Helicobacter pylori infection in children with dyspepsia in comparison to histopathology. Arq Gastroenterol. 2016;53(2):108-12. [ Links ]
Correspondence:
Hazhir Javaherizadeh
Alimentary Tract Research Center and Dept. of Pediatric Gastroenterology, Abuzar Children’s Hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Email: hazhirja@ajums.ac.ir ; hazhirja@yahoo.com
Recibido: 31-08-2017
Aprobado: 12-02-2018