Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Gastroenterología del Perú
Print version ISSN 1022-5129
Rev. gastroenterol. Perú vol.38 no.2 Lima Apr./Jun. 2018
ARTÍCULOS ORIGINALES
HAP score as prognostic factor of hepatocellular carcinoma treated with transarterial chemoembolization in a Latin American center
HAP score como factor pronóstico del carcinoma hepatocelular tratado con quimioembolización transarterial en un centro de Latinoamérica
Estefanía Liza Baca1, Javier Díaz Ferrer1
1 Department of the Digestive System, Edgardo Rebagliati Martins National Hospital, EsSalud. Lima, Peru.
ABSTRACT
Introduction: Hepatocellular carcinoma (HCC) in cirrhosis is diagnosed, most of times, when it is not susceptible to curative treatment. Transarterial chemoembolization (TACE) is a palliative therapeutic option with heterogeneous results. The HAP score stratifies patients who will benefit from the first TACE. Objective: To evaluate if the HAP score is a prognostic factor of HCC treated with TACE. Materials and methods: Retrospective cohort study in cirrhotic patients with HCC and first TACE at the Edgardo Rebagliati Martins National Hospital, Lima-Peru, from June 2011 to June 20139. The HAP score was applied, mortality and survival were observed with a follow-up of 36 months. Results: We included 54 patients with age of 67.7±9.9 years, 59.3% Child-Pugh A and 40.7% Child-Pugh B, MELD score of 11±2.7; 51.9 and 40.7% were BCLC A and B, respectively; 66.7% had a single tumor and 70.4% had a predominant tumor <5cm. The HAP score classified 8, 14, 26 and 6 patients as HAP A, B, C and D, respectively. The overall survival was 19.5±11.2 months and 32.8±6.5 months for HAP A, 24.9±14.8 months for HAP B, 13.9±5.2 months for HAP C and 14±6.6 months for HAP D. There were no deaths at 12 months in HAP A. At 24 months, mortality for HAP C and D was 100%. At 36 months, the survival rate for HAP A and B was 75 and 42.9%, respectively. Conclusions: The HAP score is a useful tool to guide the management decisions of cirrhotic patients with HCC requiring TACE due to its value in predicting mortality and survival.
Keywords: Carcinoma, hepatocellular; Liver cirrhosis; Chemoembolization, therapeutic; Prognosis (source: MeSH NLM).
RESUMEN
Introducción: El carcinoma hepatocelular (CHC) en cirrosis es diagnosticado, la mayoría de veces, cuando no es susceptible de tratamiento curativo. La quimioembolizacón transarterial (QETA) es una opción terapéutica paliativa con resultados heterogéneos. El HAP score estratifica a los pacientes que se beneficiarán con la primera QETA. Objetivo: Demostrar si el HAP score es un factor pronóstico del CHC tratado con QETA. Materiales y métodos: Estudio de cohortes retrospectivo en pacientes cirróticos con CHC y primera QETA en el Hospital Nacional Edgardo Rebagliati Martins, Lima-Perú, junio-2011 a junio-2013. Se aplicó el HAP score, y se observó la mortalidad y sobrevida con un seguimiento de 36 meses. Resultados: Se incluyeron 54 pacientes con edad de 67,7±9,9 años, 59,3% Child-Pugh A y 40,7% Child-Pugh B, MELD de 11±2,7; 51,9 y 40,7% fueron BCLC A y B, respectivamente; 66,7% tuvo tumor único y el 70,4% tumor predominante menor a 5 cm. Se clasificó como HAP A, B, C y D a 8, 14, 26 y 6 pacientes, respectivamente. La sobrevida general fue 19,5±11,2 meses; y 32,8±6,5 meses para HAP A, 24,9±14,8 meses para HAP B, 13,9±5,2 meses para HAP C y 14±6,6 meses para HAP D. A los 24 meses, la mortalidad para HAP C y D fue 100%. A los 36 meses, la sobrevida para HAP A y B fue 75 y 42,9%, respectivamente. Conclusiones: El HAP score es una herramienta útil que orienta al manejo del CHC tributario de QETA por su valor pronóstico de mortalidad y sobrevida.
Palabras clave: Carcinoma hepatocelular; Cirrosis hepática; Quimioembolización terapéutica; Pronóstico (fuente: DeCS BIREME).
INTRODUCTION
El Hepatocellular carcinoma (HCC) is a complication of cirrhosis that is now seen most frequently, but most of the time it is detected when curative treatment is no longer feasible (1,2).
Currently, the most accepted HCC classification is the one proposed by the Barcelona Clinic Liver Cancer (BCLC) group because it includes prognostic variables related to tumor status, liver function and health status, making it an integral staging system. In addition, since its initial publication in 1999, it relates the stage to the treatment strategy in a dynamic way that evolving over time due to the discovery of prognostic factors and new modalities of treatment, a fact that has been reflected in the updates made In 2003 with the incorporation of BCLC 0 (very early HCC) and transarterial chemoembolization (TACE) for intermediate HCC, and a second modification in 2008 with the incorporation of sorafenib as a first-line treatment option in advanced tumors. The BCLC classification was initially supported by the EASL, and subsequently by the AASLD guidelines for HCC management and is currently used in Peru (1,3).
TACE has been shown to be the treatment of choice for intermediate stage hepatocellular carcinoma achieving an overall survival from 16 months without intervention to 20 months after the procedure (1,3-5). Nevertheless, given the different possible clinical scenarios, influenced by characteristics related to the tumor and liver functional status, the results are variable, even reaching to observe lower than expected survivals without treatment and increased morbidity associated with the procedure (6-9).
Therefore, there was a need to create a predictive score for success or failure of the TACE in terms of overall survival, so that, several groups have published tentative scores, which are still pending to be validated in different populations and to see their applicability (10-12). Of all of them, the Hepatoma Arterial Embolization Prognostic score (HAP score) has been the most studied, since it is a simple score and easy to apply, with good results in distinguish which patients will actually achieve the expected overall survival after a TACE, avoiding procedure-related morbidity and decrease in overall survival, in those who have an unfavorable HAP score (10). An Asian (13) and French (14) group has already validated and adapted the HAP score to their reality, reproducing the results found in the original study, but there are no studies in South America that support these results, so far.
The aim of this study was to evaluate if the HAP score is a prognostic factor of HCC treated with TACE in a Latin American center.
MATERIALS AND METHODS
An observational, retrospective cohort study was performed at the Edgardo Rebagliati Martins National Hospital, Lima, Peru. We reviewed 123 medical records of in-patients in the Liver Unit from June 2011 to June 2013 with ICD-10: C22 at hospital discharge. Twenty-eight patients were excluded because they had HCC, cholangiocarcinoma or metastatic tumor and were non-cirrhotic, and 41 cirrhotic patients due to having HCC in different stages not treated with TACE or who underwent second or third TACE session. Finally, 54 cirrhotic patients with HCC who had their first session of TACE with doxorubicin, with a dynamic abdominal tomography or magnetic resonance and alphafetoprotein (AFP) within 3 months prior to the procedure and a liver function test within the 48 hours prior to HAP score determination, were included.
The HAP score was applied to each of the patients, considering that albumin <3.6 g/dl, bilirubin >1 mg/ dl, AFP >400 ng/ml and a dominant tumor size >7 cm gave 1 point to the score if they were present, classifying HAP A = 0 points, HAP B = 1 point, HAP C = 2 points, HAP D >2 points. Follow-up was until 36 months after TACE or until the patient died if it was before that period.
Data processing was performed using IBM SPSS Statistics 24, using frequency descriptive statistics and contingency tables for the calculation of mortality and survival according to the HAP score obtained. We also did a subanalysis without cirrhotics Child-Pugh B8 for eliminating any confounding factor to the results.
Our work was approved by the Committee of the Edgardo Rebagliati Martins National Hospital. Because the retrospective nature of the work, we did not obtain informed consent.
RESULTS
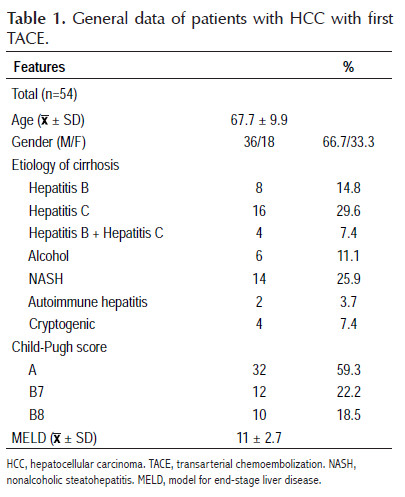
Fifty-four patients diagnosed with HCC who underwent the first TACE were included in the study whose baseline characteristics are summarized in Table 1. The mean age was 67.7 ± 9.9 years, with a range of 36 to 85 years, the majority were male (66.7%), hepatitis C was the mainly cause of cirrhosis followed by NASH and hepatitis B, 59.3% were Child-Pugh A and 40.7% Child-Pugh B, there were no patients with Child-Pugh C, and the mean MELD score was 11±2.7.
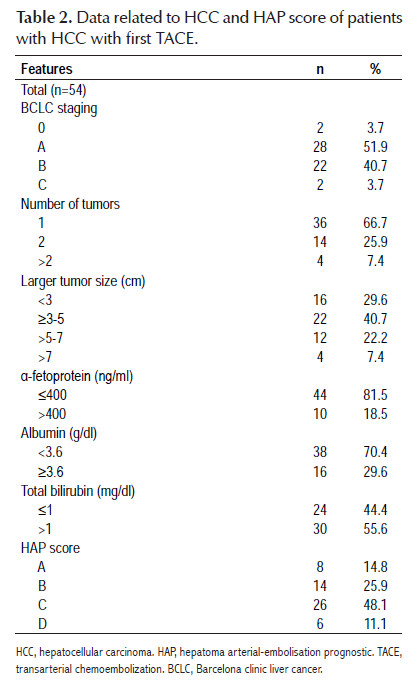
Table 2 shows data related to HCC and HAP score. Fifty-nine and 40.7% of HCC had a staging BCLC A and B, respectively. In 66.7% of cases, were solitary tumors. In 70.4% of patients, the diameter of the tumor, or of the largest tumor in the case of multinodular tumors, was found to be less than 5 cm. The HAP score was calculated in all patients and 8, 14, 26 and 6 patients were classified as HAP A, B, C and D, respectively, specifically taking into account that 40.7% of them were
HAP A or B, the group with better survival outcomes after the first TACE.
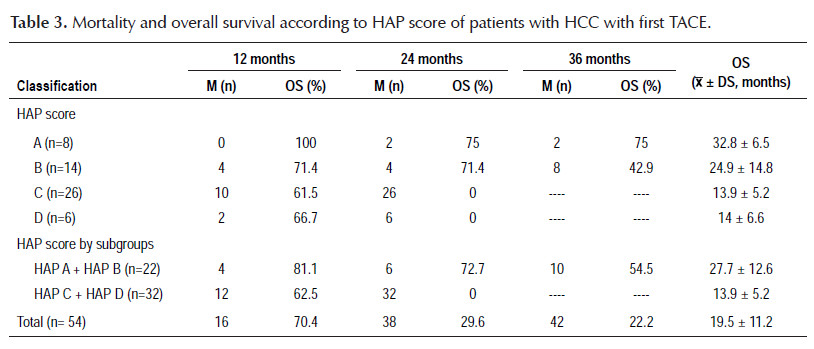
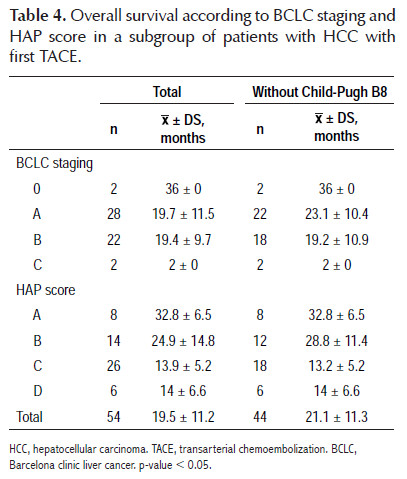
The results regarding mortality and survival following the first TACE are shown in Tables 3 and 4. Overall survival was 19.5±11.2 months; after stratification according to HAP score, overall survival was 32.8±6.5 months for HAP A, 24.9±14.8 months for HAP B, 13.9±5.2 months for HAP C and 14±6.6 months for HAP D. There was no mortality at 12 months within HAP A; when HAP A and B, and HAP C and D were grouped, it was observed that mortality within 12 months was higher for the second subgroup (18.2 versus 37.5%). The most remarkable data is that at 24 months, the mortality for HAP C and D was 100%, with an overall survival of 13.9±5.2 months and a range of 2 to 21 months. Only HAP A and B showed survival at 36 months after the first TACE, being 75 and 42.9%, respectively, with a range of 23 to 36 months for HAP A. On the other hand, it was decided to perform an overall survival sub analysis according to the BCLC stage and HAP score without considering patients with Child- Pugh B8 (8 patients), to eliminate any confounding factor, finding a slight improvement in overall survival of 21.1±11.3 months, observing a greater difference in BCLC A (23.1±10.4 versus 19.7±11.5 months) and in HAP B (28.8±11.4 versus 24.9±14.8 months).
The sensitivity, specificity, positive predictive value and negative predictive value of the HAP score was 75, 47, 32 and 82% at 1 year; 84, 100, 100 and 83% at 2 years; and of 76, 100, 100 and 55% at 3 years (data not shown).
DISCUSSION
In the BCLC classification, B or intermediate stage is treated with TACE (1,3-5), however, in our study, TACE was not only used in the intermediate stage, but also in early stages (51.9% of the patients were BCLC A and 40.7% were BCLC B), due to the fact that in the initial stages other therapeutic tools could not be used for reasons such as advanced age for liver transplantation, technical problems for proper ethanolization and lack of radiofrequency ablation in our hospital; in addition there were two elderly patients with BCLC 0, one with multiple comorbidities and the other refused any surgical intervention, and two cases with BCLC C whose staging was given by the health status. However, this did not have a significant impact on our results.
In 2013, Kadalayil et al. created the HAP score, a simple score that stratifies patients with HCC who will receive their first TACE, and uses albumin <3.6 g/dl, bilirubin >1 mg/dl as variables related to liver function, and AFP >400 ng/ml and single tumor size or the largest tumor diameter in case of multinodular tumors >7cm as HCC-related variables, and gives 1 point to the score if present, classifying as HAP A = 0 points, HAP B = 1 point, HAP C = 2 points, HAP D >2 points, with an average survival of 27.6, 18.5, 9.0 and 3.6 months, respectively, concluding as a favorable HAP to group A and B, which supports the realization of the TACE, and an unfavorable HAP to group C and D, which suggests avoiding TACE (10).
The results of our study show that 81.5% of the patients had AFP <400 ng/ml, 70.4% had serum albumin <3.6 g/dl, 55.6% had total serum bilirubin > 1mg/dl and there were only four cases in which the single tumor or the diameter of the largest tumor in case of multinodular tumors, was greater than 7 cm; these data show that the stratification of our patients according to the HAP score revolved around the liver functional status in most of them, and not only that, but more than 2/3 of them had the variable with the most negative influence in the prognosis as reported by Kadalayil et al, who found albumin <3.6 g/dl as the main prognostic variable after TACE (HR: 3.03, 95% CI:1.62-5.69), followed by the dominant tumor >7 cm (HR: 2.51, 95% CI: 1.22-5.19), AFP >400 ng/ml (HR: 2.50, 95% CI: 1.24-5.04) and finally bilirubin >1 mg/dl (HR: 2.21, 95% CI: 1.07-4.56) (10).
Although in our study population, 59.3% of cirrhotic patients were Child-Pugh A with a MELD score of 11±2.7, and that the majority of HCC were BCLC A; 59.2% had an unfavorable HAP score. This finding is of great relevance because it shows that isolated variables, such as the Child-Pugh score, does not correlate correctly with the expected final result after performing a TACE because our results show that a similar number of patients with similar characteristics had a lower survival. We decided to look deep into the survival findings in an ideal subgroup of patients, eliminating patients with Child-Pugh B8 from the analysis, not really finding a large impact on overall survival except prolongation of 2 months more than the group in general, the difference being greater in those who were BCLC A. This means that, despite the fact that TACE was performed in a controversial subgroup of patients, its presence had no significant negative effect on survival, so we can postulate that factors beyond Child-Pugh score influence the prognosis of this subgroup of patients. We thought that prognostic variables of the HAP score as a whole allow to correctly evaluate and stratify cirrhotic patients with HCC before TACE and make a better prediction of survival in the different subgroups, despite the similar liver function.
Overall survival was 19.5±11.2 months, consistent with what was mentioned by the EASL in the HCC guide for 2012, which considers an increase in patient overall survival with HCC BCLC B from 16 months without intervention to 20 months after TACE (1,5,9). Nevertheless, our patients were mostly BCLC A, suggesting that they have lower survival rates than those reported worldwide. When we analyzed the subgroup of patients without Child-Pugh B8 we found an improvement in overall survival for this stage from 19.7±11.5 months to 23.1±10.4 months.
The overall number of deaths reported in our study was 42 at 3-year follow-up, possibly correlating with the number of patients with unfavorable HAP score. Mortality after TACE was directly proportional to HAP score and survival time had an inverse association, consistent with the study of Kadalayil et al. (10). Mortality was zero in HAP A at 12-month follow-up and reached 38% in HAP C during the same period, not being very different from that found in HAP D, but the patient numbers in both groups may account for this difference. However, one of the most relevant findings that would partially justify the application of HAP score was that patients with HAP C and D had a 100% mortality within 24 months after TACE, with an overall survival of 13.9±5.2 months and 14±6.6 months, respectively, and when the joint analysis of both groups was performed, the overall survival was 13.9±5.2 months, lower than expected without any intervention, results that are similar in the study Kadalayil et al. (10), therefore, according to our findings, these patients should not be treated with TACE. Another finding that probably will encourage the use of HAP score was to find that HAP A and B had a 36-month survival of 75 and 42.9%, respectively, with an overall survival of 32.8±6.5 months and 24.9±14.8 months, respectively, better results than those reported by Kadalayil et al. who found an overall survival of 25.5 and 18.1 months, respectively (10) and when these subgroups are analyzed together, a 3-year survival of 54.5% is obtained with an overall survival of 27.7±12.6 months. These promising results may be influenced by the number of patients, the stage of the tumor, and the characteristics of the tumor, which in our study were favorable in most patients. In order to reduce possible survival biases that can be considered given the nature of the study, the overall survival subanalysis performed without Child-Pugh B8 patients is more encouraging, since there was a better overall survival in HAP B, with a difference of only 4 months compared to HAP A, in addition, it was observed that the survival in the subgroup with unfavorable HAP was the same. This means that the HAP score is useful in itself to discriminate those who will have worse survival rates independently of Child-Pugh scores, which are those in the subgroups HAP C and D. With the application of HAP score, a better selection of the candidates for TACE can be made in terms of post intervention survival.
Our study tries to eliminate possible confounding factors and reveal slightly better results than Kadalayil et al. (10). In the same way other authors like Pinato et al. have applied the HAP score in European and Asian populations, modifying and validating the score for these groups (13); similarly, Adhoute et al. reproduce and validate it in a French population, finding a good correlation between the HAP scores and survival rates post TACE (14).
Our study has several limitations: its retrospective nature, a relatively small number of patients, being performed in a single center, and utilizing a score that has not been validated in our country. Despite the limitations, this paper outlines that the HAP score applied in our setting can be a useful tool for the management of the cirrhotic patient with HCC are being considered for TACE. We recommend the validation of the HAP score for later inclusion within the management algorithm for HCC in our country.
In conclusion, the HAP score is a useful tool to guide the management decisions of cirrhotic patients with HCC requiring TACE due to its value in predicting mortality and survival. The HAP A and HAP B groups would benefit from the first TACE because these patients will have a better survival. Patients with HAP C and HAP D scores will not benefit from TACE and this palliative procedure is discouraged in this subgroup.
Conflict of interests: None.
Financial support: None.
BIBLIOGRAPHIC REFERENCES
1. European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908-43. [ Links ]
2. Bustíos Sánchez C, Díaz Ferrer J, Román Vargas R, Dávalos Moscol M, Zumaeta Villena E. Características clínicoepidemiológicas del carcinoma hepatocelular y su tratamiento en el departamento del aparato digestivo del HNERM ESSALUD. Rev Gastroenterol Peru. 2009;29(1):17-23. [ Links ]
3. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-2. [ Links ]
4. Habib A, Desai K, Hickey R, Thornburg B, Lewandowski R, Salem R. Locoregional therapy of hepatocellular carcinoma. Clin Liver Dis. 2015;19(2):401-20. [ Links ]
5. Imai N, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Hayashi K, et al. Transarterial chemoembolization for hepatocellular carcinoma: A review of techniques. World J Hepatol. 2014;6(12):844-50. [ Links ]
6. Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol, 2015;62(5):1187-95. [ Links ]
7. Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131(2):461-9. [ Links ]
8. Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62(5):1187-95. [ Links ]
9. Hao JF, Zhang LW, Bai JX, Li YJ, Liu JN, Zhang XL, et al. Incidence, risk factors, and prognosis of acute kidney injury following transarterial chemoembolization in patients with hepatocellular carcinoma: A prospective cohort study. Indian J Cancer. 2014;51(6):3. [ Links ]
10. Kadalayil L, Benini R, Pallan L, O'beirne J, Marelli L, Yu D, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24(10):2565-70. [ Links ]
11. Hucke F, Pinter M, Graziadei I, Bota S, Vogel W, Müller C, et al. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J Hepatology. 2014;61(6):1287-96. [ Links ]
12. Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, et al. A prognostic score for patients with intermediate-stage hepatocellular carcinoma treated with transarterial chemoembolization. PloS One. 2015;10(4):e0125244. [ Links ]
13. Pinato DJ, Arizumi T, Allara E, Jang JW, Smirne C, Kim YW, et al. Validation of the hepatoma arterial embolization prognostic score in European and Asian populations and proposed modification. Clin Gastroenterol Hepatol, 2015;13(6):1204-8. [ Links ]
14. Adhoute X, Penaranda G, Castellani P, Perrier H, Bourliere M. Recommendations for the use of chemoembolization in patients with hepatocellular carcinoma: Usefulness of scoring system? World J Hepatol. 2015;7(3):521-31. [ Links ]
Correspondencia:
Estefanía Liza Baca
E-mail: estefania.liza@gmail.com
Recibido: 15-03-2018
Aprobado: 28-05-2018