Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Gastroenterología del Perú
Print version ISSN 1022-5129
Rev. gastroenterol. Perú vol.38 no.2 Lima Apr./Jun. 2018
ARTÍCULO DE REVISIÓN
Management of pancreatitis and pancreatic: fluid collections
Manejo de pancreatitis y colecciones fluidas pancreáticas
Michel Kahaleh1
1 Depar tment of Medicine, Rober t Wood Johnson Medical School Rutgers, The State University of New Jersey. New Brunswick, New Jersey, United States.
ABSTRACT
Acute pancreatitis is a constant management challenge, especially with peripancreatic collection that are one of the most common complications; after the first surgical attempts that had high mortality, there had to be a new approach based in decades of acquired knowledge in physiopathology added to the development of endoscopic intervention techniques and the evolution of endoscopic devices help to establish less invasive and conservative management. This review allows us to know the last advances in the management of acute pancreatitis, pancreatic pseudocyst and walled off necrosis, determined the right time for the management to become more invasive, even considering surgery at a final stage. It also reviews the different types of drainage of peripancreatic collections and the accessories currently in use.
Keywords: Pancreatitis, Pancreatic pseudocyst; Pancreatitis, acute necrotizing (source: MeSH NLM).
RESUMEN
La Pancreatitis Aguda nos plantea un reto constante en su manejo teniendo a las colecciones líquidas peri pancreáticas como una de las complicaciones más frecuente ; inicialmente de manejo quirúrgico con una alta mortalidad, fue necesario replantear este enfoque en base a los conocimientos adquiridos durante décadas sobre su fisiopatología, que sumado al desarrollo de las técnicas de intervención endoscópica y evolución de los dispositivos endoscópicos permitió establecer manejos menos invasivos y conservadores. Esta revisión nos permite conocer los últimos avances en el manejo de la pancreatitis aguda, seudoquiste pancreático y necrosis encapsulada; determinando en que momento nuestro manejo debe tornarse más invasivo hasta llegar a la cirugía. Haciendo una revisión en los diferentes tipos de drenaje de las colecciones peri pancreáticas y los diferentes accesorios utilizados hasta el momento.
Palabras clave: Pancreatitis; Pseudoquiste pancreático; Pancreatitis aguda necrotizante (fuente: DeCS BIREME).
INTRODUCTION
Pancreatic fluid collections are a frequent complication of pancreatitis. It is estimated that 5-15% of pancreatitis episodes are complicated by development of pseudocysts (1). Fifteen percent of pancreatitis episodes are complicated by pancreatic necrosis, and approximately 33% (range 16-47%) of those with necrosis are complicated by infected necrosis (2).
Management of these collections can pose a challenge. Traditionally, the management has primarily been surgical. However, with new understanding of the pathophysiology paired with new technological advancements, the pendulum has swung towards an emphasis on a minimally invasive approach with a progression to more invasive options as necessary.
MANAGEMENT OF ACUTE PANCREATITIS
EARLY HYDRATION
Acute pancreatitis can result in severe hypovolemia due to limited oral intake, vomiting, third spacing, and diaphoresis. In addition to these macro- circulatory adverse effects, acute pancreatitis through a combination of microangiopathic effects reduces pancreatic blood flow activating a number of cascades resulting in pancreatic hypoperfusion, cell necrosis and death (3). Therefore, early aggressive hydration is the foundation of treatment in the early stages of pancreatitis. Numerous studies have proven that early aggressive hydration in acute pancreatitis is both effective and reduces complications by restoring both the macro and micro-circulatory systems (4-6). However, there are limited surrogate serological markers to follow to analyze response to hydration. Hemoconcentration has been shown to increases morbidity associated with pancreatitis (7). Current data demonstrate that non- inflammatory markers, hematocrit, BUN and creatinine, are the most used and widely recommended markers to follow during hydration (8-10).
Timing
Early (first 24 hours) aggressive hydration is key in the initial management of acute pancreatitis. Gardner et al demonstrated that patients who received greater than 33% of their total hydration in the first 24 hours were significantly less likely to suffer in-hospital complications from pancreatitis (11). This was replicated in another study by Warndorf et al. (6).
Type
In an elegant randomized control trial, Wu et al. (10) demonstrated that Lactated Ringers results in significantly less inflammation and morbidity than (0.9%) normal saline. This was due in part to the more pH balanced nature of Lactated Ringers compared to normal saline.
In conclusion, early aggressive hydration with lactated ringers and close surrogate marker (HCT, BUN, Cr) monitoring is paramount in the appropriate initial management of acute pancreatitis.
ANTIBIOTIC THERAPY
Infectious pancreatic and extra-pancreatic complications can occur as a result of pancreatitis. The systemic inflammatory response syndrome (SIRS) associated with sterile pancreatitis may be indistinguishable from infections associated with pancreatitis. Therefore, antibiotics should be used when an infection is identified or clinically suspected, but not in all cases of pancreatitis (2). When extra- pancreatic infectious complications are identified, antibiotic therapy should be tailored to such infection. The paradigm of antibiotic use in pancreatitis is to identify and prevent infected pancreatic necrosis, a cause of significant morbidity and mortality (12). Because of the consistency of pancreatic necrosis, few intravenous antibiotics effectively penetrate. A meta- analysis of 11 randomized control trials examining the role of antibiotics in pancreatitis failed to demonstrate a benefit in sterile necrosis (13) with a calculated NNT 1,429 for one patient benefit, regardless of the severity of pancreatitis (14). Rather than preventing infection, antibiotics are now used to treat established infected pancreatic necrosis. Recent studies have demonstrated that antibiotics alone may resolve infected pancreatic necrosis (15,16). However, these patients should be followed closely to determine whether definitive drainage is necessary. When antibiotics are necessary, those proven to have the highest pancreatic penetration (carbapenem, quinolones, metronidazole and high- dose cephalosporines) should be preferred (9,14).
INDICATION FOR ERCP
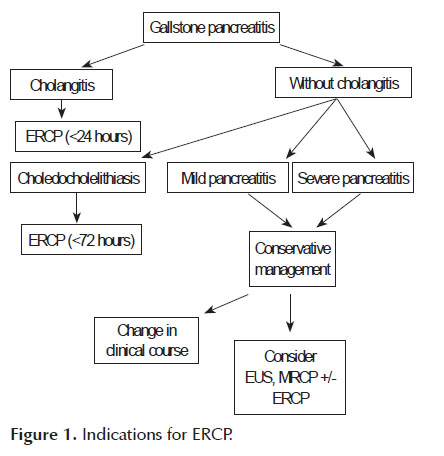
Gallstone disease can account for up to fifty percent of acute pancreatitis cases (14,17). Most gallstones that cause pancreatitis pass into the duodenum and are lost in the stool (14,18). However,a small minority of patients have obstructing gallstones that may only be relieved by ERCP. Early ERCP (<24 hours) with biliary sphincterotomy and stone removal has been proposed to ameliorate the course of pancreatitis in these patients. The current indications for ERCP in gallstone pancreatitis,as reviewed by Fogel et al. (19), include suspected bile duct stones as the cause of pancreatitis in addition to one of the following: a) cholangitis as evidenced by fever, jaundice or sepsis, b) persistent biliary obstruction as evidenced by conjugated bilirubin >5mg/dl, c) clinical deterioration as evidenced by worsening pain, increased white blood cell count, or worsening vital signs, or d) choledocholelithiasis on imaging.
The severity of gallstone pancreatitis is not an indication for immediate ERCP. Tse et al. (20) in a Cochrane database review of 5 of the largest RCTs (21- 25) including 644 patients compared early conservative management of pancreatitis to early ERCP for gallstone pancreatitis. Although there was heterogeneity in the various studies, the authors concluded that the indication for ERCP should be based on evidence of cholangitis or biliary obstruction rather than on the severity of pancreatitis alone. However, they also suggested that the lack of uniform sphincterotomy during ERCP in these trials limits general application of these results to all cases of gallstone pancreatitis (26). It is therefore suggested that patients with severe pancreatitis who are managed conservatively should be followed closely for signs and symptoms of clinical deterioration and should undergo further imaging (endoscopic ultrasound or magnetic resonance cholangiopancreatiography) to rule out biliary obstruction when clinical deterioration occurs. In summary, we propose that the following algorithm should be considered in the consideration of ERCP in gallstone pancreatitis (Figure 1).
CLASSIFICATION OF PANCREATIC FLUID COLLECTIONS
Correctly classifying PFCs is critical for optimizing treatment and management. The first widespread classification system was developed in 1993 by an international consensus meeting in Atlanta, Georgia and became referred to as the Atlanta Criteria (27). This criteria classified pancreatic fluid collections as acute or chronic collections, with chronic collections being further divided intopancreatic necrosis, pseudocysts, and pancreatic abscesses.
However, with improving pathophysiologic understanding and improving diagnostic tools, it became clear that a more detailed organizational system was required. More specifically, distinguishing between collections containing fluid alone versus those arising from necrosis and/or containing solid components. As such, a new classification system was developed known as the revised Atlanta criteria (28). Similar to the original Atlanta Criteria, PFCs are classified as acute (<4 weeks after the pancreatitis episode) or chronic (>4 weeks after the pancreatitis episode). However, in the revised criteria, both acute and chronic collections are further subdivided based on the presence of necrosis within the collection. Acute collections are divided into: acute peripancreatic fluid collections (APFC) and acute necrotic collections (ANC); chronic fluid collections are divided into: pseudocysts or walled-off pancreatic necrosis (WOPN). These new classifications are important because the treatment and management varies depending on the type of collection.
ENTERAL FEEDING
The first step in the management of any PFC is ensuring adequate nutritional support. In mild to moderate acute pancreatitis, oral feeding can be initiated when symptoms are controlled. In severe cases, patients have traditionally been kept nil per os (npo) due to concerns for worsening pancreatic inflammation if normal pancreatic digestion were to be enacted during oral intake (29). However, prolongednpo in the catabolic stress state of pancreatitis leads to a negative nitrogen balance and nutritional deficiency that became recognized to be associated with a higher mortality rate due to loss of function and structural integrity of vital organs (30). As a result, total parental nutrition (TPN) became the standard of care in patients with severe acute pancreatitis in an attempt to avoid pancreatic stimulation while still providing nutritional support (29,30).
ENTERAL FEEDING VERSUS TPN
This approach was questioned when studies began showing that complete bowel rest is associated with intestinal mucosal atrophy leading to increased intestinal permeability and bacterial translocation (14). Furthermore, a metabolically deprived gut absorbs endotoxins and other bacterial products stimulating endogenous cytokines which increases the likelihood of nosocomial infections, sepsis, and organ failure (31). The use of TPN was further called into question with the emergence of data showing that enteral feeding distal to the ligament of Treitz causes negligible pancreatic stimulation and therefore may be safe in patients with severe pancreatitis (32).
In 2010, the Cochrane Colloboration published their results of a meta-analysis comparing randomized trials of enteral nutrition versus TPN in patients with severe acute pancreatitis (30). Enternal nutrition was associated with a significant reduction in mortality, multi-system organ failure, and systemic infections with a trend towards shorter length of hospital stay. Based on these findings, enteral nutrition was recommended as the standard of care for nutritional support in acute pancreatitis (30).
In addition to improved morbidity and mortality rates, enteral nutrition is associated with a lower overall cost compared to TPN. In a study of 24 patients with severe acute pancreatitis, enteral nutrition was associated with savings of $5,553.06 per patient (p=0.08). Though not statistically significant, there was a medium to large effect size (d=0.61) suggesting that the difference between the two groups would likely have been significant in a larger sample size (33).
EARLY VERSUS LATE ENTERAL FEEDING
The timing of initiation of enteral feeding in severe acute pancreatitis has been debated. In a recent meta-analysis, patients receiving early initiation of enteral nutrition (defined as within 48 hours of admission) had significantly lower rates of infectious complications (OR 0.45; 95% CI 0.15-0.77, p<0.05), organ failure (OR 0.27; 95%CI 0.14- 0.50, p<0.05), length of hospitalization (mean difference -2.18 (days); 95%CI -3.48-(-0.87); p<0.05, and mortality (OR 0.31; 95%CI 0.14-0.71, p<0.05) compared to those with delayed enteral nutrition or TPN (34). However, the exact time at which enteral feeding should be initiated is not yet established.
NASOJEJUNAL VERSUS NASOGASTRIC ENTERAL FEEDING
Though enteral nutrition distal to the ligament of Treitz is thought to decrease pancreatic stimulation, placement of a nasojejunal tube requires endoscopy for placement and is more cumbersome than a nasogatric tube which can be placed bedside. Studies have been performed to evaluate the safety of nasogastric feeds compared to nasojejunal feeds. A meta-analysis of these studies showed no difference in mortality and tolerance between the two types of feeding; however, this analysis was limited by the small sample size (157 patients in the 3 studies included for analysis) and the lack of verication of placement of the nasojejunal tube distal to ligament of Treitz in two of the three studies (35). A recent non-inferiority trial of 78 patients randomized to nasogastric or radiologically-confirmed nasojejunal feeding was recently published, showing non-inferiority of nasogastric feeds. However, there was a higher rate of infectious complications, need for surgical intervention for infected necrosis, and mortality in the nasogastric feeding group (36). A prospective, randomized controlled trial evaluating nasogastric versus nasojejunal feeding called the Study on Nutrition in Acute Pancreatitis is currently underway, which will provide further evidence on this subject. Until more high-quality data is available, nasojejunal feeding remains the preferred route of enteral nutrition.
ENTERAL FEEDING FORMULATIONS
Enteral nutrition is available in a variety of formulations, including standard, elemental, and semi- elemental with the latter two more commonly used based upon the assumption that they result in less pancreatic stimulation. Standard enteral formulas are, however, significantly cheaper and proven effective (37). Windsor et al randomized patients with severe acute pancreatitis to TPN versus standard enteral formulas (Osmolite) and found that patients receiving standard enteral formulas had improved clinical outcomes compared to those receiving TPN, including decreased rates of systemic inflammatory response syndrome, sepsis, and multi-system organ failure (38). Makola et al also examined the efficacy of enteral formuals in acute pancreatitis and demonstrated that it is associated with an improvement in the severity of pancreatitis, a higher albumin, and a trend towards a normal BMI (37).
INDICATIONS FOR DRAINAGE OF PFCS
In the initial Atlanta criteria, PFCs were recommended for drainage based on the presence of symptoms and/or complications such as abdominal pain, gastrointestinal obstruction, vascular compression, biliary obstruction, or infection, as well as on the size of the collection. However, in the revised criteria, size alone does not necessitate treatment; only symptomatic PFCs are recommended for drainage. Another recent departure from the classic approach is proceeding with intervention based not exclusively on culture or Gram stain verified infection, but also on imaging (the presence of air in the collection on CT) and/or clinical criteria (failure to improve with aggressive supportive care, or even failure to thrive). The optimal treatment window is ideally four weeks after the initial event to allow peripancreatic fluid collections to wall-off and areas of tissue necrosis to demarcate. Historically, drainage has been managed via surgical techniques, but with the advent of newer and more advanced endoscopic tools and expertise, and an associated reduction in health care costs, minimally invasive endoscopic drainage has become the preferable approach.
PANCREATIC PSEUDOCYSTS
As described in the revised Atlanta criteria, a pseudocyst is an encapsulated fluid collection, without the presence of solid debris, that develops as a consequence of pancreatitis a minimum of 4 week after the initial injury (27).
SURGICAL DRAINAGE
Surgical cystgastrostomy involves an open or laparoscopic procedure in which an anastomosis is created between the lumen of the cyst cavity and the stomach or small bowel using suturing or stapling devices. Historically, surgical drainage was an efficacious therapy, with published pseudocyst recurrence rates between 2.5-5% post-drainage, but complication rates approaching 30% in some studies (39). As endoscopic therapies emerged, initial studies comparing surgical cystgastrostomy to endoscopic cystgastrostomy showed grossly equivalent success rates, defined as pseudocyst resolution, and comparable complication rates (40,41). However, as endoscopic techniques improved, endoscopic therapy became the preferred initial treatment approach. A randomized comparative trial by Varadarajulu et al. looking at surgical versus endoscopic cystgastrostomy found that while the two techniques yielded similar technical success and complication rates, endoscopic therapy was associated with a shorter hospital stay, a lower overall cost, and better mental health and physical health component scores among patients (42).
PERCUTANEOUS DRAINAGE
Percutaneous drainage involves placement of an external drainage catheter into the pseudocyst using real-time imaging guidance, usually with computed tomography (CT) or ultrasound (US) with fluoroscopy. Initial studies comparing surgical drainage to percutaneous drainage found both procedures to be efficacious (43,44). However, more recent comparative studies have generally favored percutaneous drainage (45), with some studies even demonstrating a mortality benefit (46). A recent study directly comparing percutaneous versus endoscopic management retrospectively reviewed 81 patients. This study found equal technical success rates and adverse events rates between the techniques, but a decreased re-intervention rate, a shorter hospital stay, and a decreased number of follow-up abdominal imaging studies among patients drained endoscopically (47).
CONVENTIONAL TRANSMURAL DRAINAGE (CTD)
Conventional transmural drainage was the endoscopic procedure of choice to drain PFCs in the early era of endoscopic PFC management. This procedure consists of endoscopically visualizing the PFC bulge in the gastric wall, creating a fistulous tract between the pseudocyst cavity and the gastric lumen using a seldinger technique, advancing a guidewire into the pseudocyst cavity, dilating the tract, and finally deploying one or more plastic stents to secure apposition and allow for continuous drainage (48).
This concept was first introduced into the medical literature in 1975 in a case report by Rogers et al. (49). It was expanded upon by Kozarek et al. in 1985 in a case series of 4 patients who underwent endoscopic cystgastrostomy needle decompression (50) and by Cremer et al in 1986 in which they described 13 patients who underwent cystgastrostomy with transnasal drain placement (51). The first large series evaluating this technique was published in 1989 and consisted of a 7-year follow-up study of 33 patients who underwent endoscopic cystgastrostomy or cystduodenostomy with a success rate of 82%, recurrence rate of 12%, and complication rate of 2% (52). In 1995, Binmoeller et al. published a similar study of 53 patients with a success rate of 87%, recurrent rate of 21%, and complication rate of 11% (53). A series of subsequent studies from the early 2000s demonstrated similar results, reporting success rates between 70-100% and complication rates ranging from 2-40%, mainly bleeding, perforation, and infection due to stent occlusion or migration (52-62).
One of the limitations of this technique was the need for the PFC to be bulging into the gastric wall. It is estimated that no bulge is present in 42-48% of PFCs, limiting the efficacy and safety of this technique in almost half of all cases (63). However, with the incorporation of echoendoscopy, this limitation was able to be overcome.
EUS-GUIDED TRANSMURAL DRAINAGE
The useof EUS in pseudocyst drainage provides endoscopists with the ability to identify and avoid vascular structures between the cyst and the gastric lumen, to measure the distance between the lumen and the cystic lesion and ensure that adequate apposition can be obtained, to localize non-bulging pseudocysts that are otherwise unidentifiable using endoscopy alone, and to confirm the lack of solid or necrotic components within the pseudocyst cavity. This technique first emerged in the medical literature in 1992 by Grimm et al. (64) and 1996 by Wiersema et al. (65), both of whom described a single case of successful endoscopic pseudocyst drainage using an echoendoscope. Several larger case serieslooking at 27 patients (53) and 35 patients (66) documented success rates of 78% and 89% with complication rates of 7% and 4%, significantly lower than with CTD. Since then, a multitude of studies have validated these initial findings, with early studies quoting success rates ranging from 80%-100% and complication rates averaging around 10%, mainly bleeding and perforation (48,53,58,63,66-70).
More recent studies have further subdivided pancreatic pseudocysts into simple versus infected pseudocysts. Sadik et al. noted a 94% success rate and 5% complication rate in simple pseudocysts versus 80% success rate and 30% complication rate in infected pseudocysts (71). Similarly, Varadarajulu et al. found a 93.5% success rate and 5% complication rate versus a 63% success rate and 16% complication rate in sterile versus infected pseudocysts (72). This suggests that alternate means of drainage may be beneficial when infection is present.
Several studies have directly compared EUS-guided PFC drainage to CTD. A study by Kahaleh et al. (48) showed equal efficacy and safety between the two techniques when conventional drainage was used for bulging lesions and EUS-guided drainage was used for all other lesions. Subsequently, two prospective, randomized studies by Varadarajulu et al. (73) and Park et al. (74) found significantly higher technical success rates with EUS-guided drainage, and a trend towards a better safety profile although statistical significance was not reached.
FULLY COVERED SELF-EXPANDING METAL STENTS (FCSEMS)
Fully-covered self-expanding metal stents (FCSEMS) offer a variety of advantages over traditional plastic stents. Firstly, they allow for a larger drainage lumen, which decreases the risk of stent occlusion and theoretically the need for repeat procedures. And secondly, they allow for shorter procedure times since they require a single access of the cyst for deployment, rather than the multiple access points required for the deployment of multiple plastic stents.
A study by Penn et al. (75) looked at 20 patients with symptomatic pancreatic pseudocysts which were drained under EUS guidance with placement of biliary FCSEMS (Wallflex; Boston Scientific, Natick, MA). They found a 100% technical success rate and a 70% rate of complete pseudocyst resolution without recurrence. Three patients experienced complications (15%) requiring surgery in 2 of the 3, and stent migration was noted in 3 patients, all of whom still achieved pseudocyst resolution. Similarly, a case series looking at 18 patients with symptomatic pseudocysts drained with FCSEMS (Wallflex; Boston Scientific) under EUS- guidance showed a 78% rate of complete pseudocyst resolution (14 patients); however, 16% of patients required surgery for ongoing sepsis or ineffective drainage (76). A case series looking at 20 patients with infected pseudocysts drained with biliary FCSEMS and/or esophageal CSEMS reported a 100% technical success rate and a complete clinical success rate of 85% (77). Another series, confirmed those findings (78).
FCSEMS with antimigratory fins (Viabil, Conmed, city, state) have also been proven efficacious. Talreja et al. (79) reported a 78% clinical success rate with complete resolution after pseudocyst drainage in 18 patients. In their series, 1 patient had stent migration, though still achieved pseudocyst resolution. Berzosa et al. evaluated the same stent for pseudocyst drainage in 5 patients and found an 83% resolution rate without recurrence at 18 weeks (80).
PLASTIC STENTS VERSUS METAL STENTS
Despite the advantages that FCSEMS hold over traditional plastic stents, direct comparison has not consistently shown them to be superior. A recent meta- analysis that included 698 patients found no difference in treatment success, adverse events, or recurrence rates between pseudocysts drained with multiple plastic stents versus metal stents (41,81). However, a more recentstudy by Sharaiha et al of 230 patients found that pseudocysts drained with plastic stents were 2.5 times more likely to report adverse events than when FCSEMS were used. Similarly, complete pseudocyst resolution was 89% with plastic stents compared to 98% with FCSEMS (82).
A NOVEL LUMEN-APPOSING METAL STENT
In 2013, a new FCSEMS received FDA approval for use in drainage of PFCs (Axios; Xlumena, city, state). The design of the stent includes two 21mm or 24mm the gold standard therapy for WOPN . Surgical flanges on either side of a 10 mm or 15 mm diameter lumen to help decrease the risk of stent migration. The first clinical data using this stent came from a study by Itoi et al in 2012 looking at 15 patients with symptomatic pseudocysts. Success rate in the trial was 100%, with zero percent recurrence at 11-month follow-up and the only complication being stent migration in 1 patient without clinical sequelae (83).
Two additional studies validated this initial reported success. A prospective study by Shah et al. (84) looking at 33 patients found a technical success rate of 91% with a pseudocyst resolution rate of 93%. Gornals et al. (85) looked at 9 patients who underwent pseudocyst drainage with placement of a LAMS and reported a technical success 89%, a pseudocyst resolution rate of 100%, and 1 significant complication (pneumothorax). Most recently, Walter et al. (86) published their data of 15 patients with a clinical success rate of 93%, resolution rate of 100%, and 1 significant complication (perforation).
In summary, pancreatic pseudocysts can be efficaciously managed endoscopically. Although conventional endoscopic drainage can be safely used for bulging pseudocysts, the majority of pseudocysts are drained under EUS-guidance to allow for safer access and a decrease in complications. Metal stents, including the newly emerged lumen-apposing metal stent, carry a clear advantage over plastic stents in pseudocyst drainage due to their increased diameter (up to 15 mm).
MANAGEMENT OF WALLED OFF PANCREATIC NECROSIS (WOPN)
Walled off pancreatic necrosis (WOPN) is a PFC that contains solid necrotic debrissurrounded by a clearly defined capsule with or without concurrent fluid (28). Although a small percentage of WOPN will resolve spontaneously, the majority of collections will require drainage.
SURGICAL DRAINAGE
Open surgical debridement has historically been (87,88) management consists of 4 principal approaches, all involving accessing the pancreatic bed but differing in the surgical approach. The standard approaches include access via the lesser sac, the gastrocolic- omentum, or trans-mesenterially through the transverse mesocolon (89). Once the necrosectomy has been performed, the options are (1): necrosectomy alongside open packing (90); (2) planed, staged re-laparotomies with repeat lavage (91); (3) closed continuous lavage of the lesser sac and retro- peritoneum (88); and (4) closed packing (92). Unfortunately, open necrosectomy is associated with high morbidity (34% to 95%) and morality (6% to 25%) rates (93-98), and a plethora of adverse events including organ failure, perforation, wound infections, hemorrhage, chronic pancreatico-cutaneous and entero-cutaneous fistulae, and abdominal wall herias (87,89,92,94,95).
With the development of laparoscopic surgery, minimally invasive procedures supplanted open debridement as the surgical option of choice. Laparoscopic debridement can be performed using 2 approaches: trans-peritoneal (anterior) or retroperitoneal (posterior) (88). The trans-peritoneal approach involves an anterior access through the stomach or the bowel to drain the collection. The retroperitoneal approach uses a mini-lumbotomy, usually left-sided, through which a laparoscope is introduced to remove the necrotic debris under direct visualization Currently, the trans- peritoneal approach is rarely used due to increased technical difficulty and the risk of contamination of the peritoneal cavity (99). The retroperitoneal approach, Video Assisted Retroperitoneal Debridment (the VARD procedure), can be performed with minimal or no gas insufflation and avoids the complications associated with severing the peritoneum (100,101). (see technique below).
Preoperative Planning
As emphasized earlier, several factors require consideration prior to embarking upon surgical necrosectomy. First, the procedure should be delayed ideally for at least one month after onset of pancreatitis, for demarcation of necrosis as well as maturation of the collection. Second, a 12 or 14 French percutaneous drain is placed with CT guidance in the peripancreatic collection via a left flank approach. Even if this is not intended as destination therapy, it is essential for the guidance of the incision for the videoscopic approach to the retroperitoneum. Cross-sectional imaging should be studied carefully with respect to external landmarks and anatomic structures (spleen, colon) adjacent to the collection. Anesthesiology should be prepared for hemodynamic consequences with appropriate invasive monitoring.
Surgical Anatomy
Visualization of the location of the collection in relationship to external landmarks is key for optimal trocar placement. The pancreas lies in front of the second lumbar vertebra in the retroperitoneal space, with the body and tail extending laterally and slightly superiorly. Preoperative imaging should be examined carefully to assess the path and location of the actual collection, which in cases appropriate for the VARD is generally in the region of the body and tail, though can extend along the left gutter inferiorly as well. The percutaneous drain provides guidance for the route to the collection; it is beneficial for this drain to not emerge in the intercostal position, and this should be communicated to the interventional radiologist.
Step-by-step illustration of procedure
The patient is placed in slight right lateral decubitus position, with the left shoulder and body oriented about 40 degrees from the horizontal (exact angle is partly determined by location of emergence of the drain). The left arm is easily managed on a well-padded armboard, and the body may be cradled on a bean bag for extra support. The knees are slightly bent with a pillow placed in between. Extremes in Trendelenberg position are generally not anticipated thus extra support at the feet or head is unnecessary. The operating table is slightly bent to allow extension of the left flank and thus more working room for trocars as needed.
Entry into the retroperitoneal space may be obtained via a small (5 cm) subcostal incision in the midaxillary line. The fascia is carefully dissected to allow entry into the retroperitoneum. The initial efflux of pus and small particulate matter is evacuated with a standard suction, and any easily grasped necrotic tissue is extracted with long forceps. A laparoscopic trocar is then placed through the incision and a 0-degree videoscope introduced. Carbon dioxide may be insufflated via the drain. Debridement is affected under direct vision with a laparoscopic forceps directly through the flank incision. Alternatively, a two-trocar method may be used. Access to the retroperitoneum may be obtained using a transparent trocar system with optical visualization with a 0-degree videoscope through the obturator. The trocar is slowly and carefully advanced along the path of the drain, which can be kept in sight along the side of the cannula. Once visual and tactile confirmation of entry into the cavity is obtained, the cavity is bluntly developed with a 5 mm suction tip. When space-occupying fluid is removed, the cavity is insufflated with carbon dioxide gas, to a pressure of 12 to 15 mmHg. A second 5 mm trocar is placed in a position determined in part by the drain location and the anatomy. One of these trocars is then ideally upsized to a 10 or 12 mm for removal of larger debris.
The cavity is then developed by gently probing, suctioning and grasping loose necrotic tissue. As in standard open necrosectomy, necrotic tissue is debrided gently and not forced, to avoid vascular and other organ injuries and preserve viable pancreatic tissue; this may appear to be incomplete but is nonetheless a wholly adequate debridement. Once the loose necrotic tissue has been removed, the cavity is copiously irrigated with sterile saline solution. The percutaneous drain is removed and surgical drains are place; in the two- trocar technique an irrigating triple lumen sump drain is placed through the larger incision, preferably under direct vision via the remaining trocar, and small drain is left through the small trocar incision. The large sump drain is used for continuous irrigation postoperatively, for 24 or more hours until the irrigant runs clear (75- 100 cc/h of normal saline). The smaller drain functions mostly as a place holder, as the larger drain suctions the majority of the fluid. As the potential for any repeat surgery fades, this drain can be removed. Some prefer to maintain the larger sump drain on suction, and as the clinical status of the patient improves and the drainage lessens, it is gradually withdrawn 1-2 cm per day. Presumably maintaining the suction during the withdrawal process facilitates the collapse of the recently evacuated tract.
In circumstances of extension of the walled off collection and necrosis across the midline or into the root of the mesentery, open surgery is necessary. Numerous variations of technique have been described and include variations on open marsupialization with relaparotomy for further debridement and packing changes, or debridement and closure over drains with continuous lavage. Approach to the retroperitoneum is typically via a generous bilateral subcostal incision; this provides excellent exposure to the lesser sac but also allows access to the inframesocolic abdomen if necessary for feeding jejunostomy or diverting ileostomy or colostomy. The layers of the abdominal wall are carefully divided and the peritoneal cavity entered. The gastrocolic ligament is divided to enter the lesser sac, which is often at least partially fused by inflammatory reaction. Careful blunt dissection facilitates entrance into the lesser sac, which harbors the walled-off collection and necrotic pancreas and peripancreatic tissues. As already described for the minimally invasive technique, the collection is drained and loose necrotic tissue is carefully removed. It is helpful to lavage the cavity with warm saline to indicate areas of loose tissue to be extracted. Careful extension into and across the midline toward the head, across to the paraduodenal space or into the pelvis may be needed based on the appearance of preoperative imaging.
At this point other procedures may be undertaken as indicated, including cholecystectomy (if the etiology is biliary), gastrostomy, tube jejunostomy, or fecal diversion (for colonic fistula). One of two courses may be followed now: packing of the cavity with moist gauze and planned second look (marsupialization), or operative placement of irrigating drains into all affected areas and closure of the abdominal wall. The first may be indicated for control of intraoperative hemorrhage. The latter carries less morbidity and allows quicker extubation as well as a lower overall morbidity rate.
Other approaches include accessing the lesser sac via the transverse mesocolon. The technique is identical to that already described, namely careful blunt removal of loose necrotic tissues and avoidance of overly aggressive debridement. Drains are left in any resultant cavities and the wound is closed. The purported advantages of this approach over the anterior approach to the lesser sac include: 1. avoidance of the potentially hazardous dissection of the transverse colon from the stomach and omentum; 2. possibly decreasing the risk of colocutaneous fistula; and 3. placement of drains in a more dependent position than the anterior approach. However, there is concern about introducing infected material from the formerly walled-off abscess into previously virgin territory, namely the inframesocolic abdomen, thus spreading local sepsis beyond its original borders. This suggests that a retro-peritoneal only technique is the most advantageous approach.
PERCUTANOUS DRAINAGE
Percutaneous drainage for WOPN involves placement of a catheter into the collection under US guidance with fluoroscopy or CT guidance. Ideally, a retroperitoneal approach is taken. After placement and aspiration of as much fluid as possible, 12French drains are left in place and irrigated with 10–20 mL of sterile saline 3 times daily. Multiplecatheters are typically required as the patient’s follow-up requires (102).
Traditionally, the success rate of percutaneous drainage alone (defined as survival without the need for additional surgical necrosectomy) ranged from 35% - 84%, with mortality rates ranging from 5.6%-34% and morbidity ranges of 11%-42%, most commonly due to pancreatico-cutaneous fistulas and pancreatico-enteric fistulas which occur in an as many as 20% of cases (103- 107). Consequently, percutaneous drainage is more often used as an adjunct therapy, often serving as the first step of a step-up approach to endoscopic or surgical drainage (87,98,103). The Dutch PANTER trial illustrated this concept by comparing open necrosectomy with a less-invasive step- up approach in 88 patients (108). In the step-up approach, patients first underwent percutaneous drainage of the collection followed by minimally invasive retroperitoneal necrosectomy if clinical improvement was not achieved. Results showed that the minimally invasive approach was associated with an overall decreased mortality rate, fewer major and long-term complications, and reduced overall healthcare costs. Of note, percutaneous drainage alone without subsequent necrosectomy was achieved only in 30% of patients.
ENDOSCOPIC NECROSECTOMY
The endoscopic technique for drainage of WOPN is called endoscopic necrosectomy. As in pseudocyst drainage, EUS is used to identify and access the collection, a wire is coiled within the cavity lumen, and the fistulous tract is created. However, unlike pseudocyst drainage, the tract is then dilated enough to allow for passage of an endoscope into the collection. Mechanical cleaning with removal of necrotic debris is then performed. Nasocystic drainage is typically performed to facilitate liquefaction of the debris and improve drainage (54).
Hydrogen peroxide (H2O2) can be used to facilitate removal of necrotic debris (15). H2O2 is infused into the cavity during endoscopy in a 1:5 or 1:10 dilution with normal saline, allowing for enhanced necrotic tissue dislodgement and debris extraction during endoscopy. The use of H2O2 has been shown to decrease procedure time, reduce complication rates, and decrease the total number of necrosectomy sessions until resolution. Some adverse events have been reported including bleeding, perforation, and self-limited pneumoperitoneum. However, these complications are rare, especially after the incorporation of carbon dioxide for peri-procedural insufflation.
The first experiences with endoscopic necrosectomy were done through the deployment of plastic stents and placement of a nasocystic drain without direct mechanical debridement. This was first described by Baron et al in 1996 (109), in which 11 patients underwent WOPN drainage with an overall success rate of 81% and a complication rate of 36% (bleeding and infection). Papachristou et al. reported similar findings in 2007 in a study of 53 patients, with an overall success rate of 81% and a complication rate of 21% (110).
Subsequent studies reported similar findings, with success rates ranging from 75%-95%, and complication rates ranging from 0 to 35%.
Seewald et al introduced the concept of dilation of the fistulous tract to allow for advancement of an endoscope into the necrotic cavity and mechanical removal of debris (111). They described a 91% WOPN resolution rate in 13 patients, with 2 patients having recurrence on 4-month follow-up necessitating surgical resection. Voermans et al. documented a 93% success rate in 25 patients, with only 2 patients requiring surgical intervention for bleeding and perforation (112). Smaller studies by Escourrou et al. (113) and Charnley et al. (114) found similar results.
The first multicenter study evaluating endoscopic necrosectomy was performed by Seifert et al. (115). In this study of 93 patients, an 80% clinical success rate was achieved with a 23% complication rate and 7.5% mortality rate. A second multicenter study was published by Gardner et al in 2011 (116) looking at 104 patients with WOPN. Successful resolution was achieved in 91% of patients, with a complication rate of 14% including 3 patients requiring surgical intervention either for bleeding or failed resolution, 5 patients dying of other causes prior to WOPN resolution, and 1 peri- procedural death due to hypotension.
FULLY-COVERED SELF-EXPANDING METAL STENTS
Biliary FCSEMS provide a larger stent lumen for drainage of WOPN but are limited in that they do not permit passage of an endoscope. Fabbri et al. published results of 2 patients with WOPN drained with biliary FCSEMS (Wallflex, Boston Scientific) (117). In 1 patient, the WOPN completely resolved; in the second patient, the stent migrated leading to widespread sepsis and need for surgical intervention. Berzosa et al. also looked at 2 patients with WOPN drained with biliary FCSEMS (GoreViable, ConMed) (80). The WOPN resolved in both patients with no recurrence after 18 weeks follow-up.
Esophageal FCSEMS have a larger lumen diameter and allow for passage of the endoscope through the lumen of the stent after deployment. The first reported case of WOPN drainage using an esophageal FCSEMS was published by Antillon et al. (118). Sarkaria et al. published results of 17 patients who underwent WOPN drainage with placement of an esophageal stent, 88% of whom demonstrated complete resolution over an average of 5 endoscopic sessions and 2 of whom ultimately required surgical intervention (119). No major complications were reported. Attam et al found similar results in 10 patients using a through- the-scope esophageal FCSEMS in which resolution was achieved in 90% of patients after an average of 3 endoscopic sessions (120). 2 patients required stent revision due to persistent infection in long-term follow- up, and 1 patient died of gastrointestinal bleeding from a pseudoaneurysm.
A NOVEL LUMEN-APPOSING METAL STENT (LAMS)
The previously mentioned LAMS (Axios, Xlumena) with a diameter up to 20 mm also allows for passage of an endoscope through the lumen of the stent into the cavity for mechanical necrosectomy. Only a small number of studies have been published specifically evaluating the use of LAMS for drainage of WOPN. Shah et al achieved WOPN resolution in 10 of 11 patients using a LAMS for drainage (84). The largest clinical experience comes from Walter et al in which they looked at 46 patients with WOPN (86). They reported a clinical success rate of 81%, with 3 patients ultimately requiring surgical intervention for persistent infection despite drainage and an overall major complication rate of 9%, all due to infection from stent occlusion and managed endoscopically. Additional multi-center studies are needed, but LAMS represent a promising advance in the endoscopic management of WOPN.
Cumulatively, these studies illustrate that while endoscopic necrosectomy is efficacious, it is a complicated procedure requiring a high-level of skill in endoscopy with complications occurring even in the most experienced of hands and requiring the presence of a strong multi-disciplinary team to be successful. The incorporation of metal stents, especially lumen apposing metal stents, that allow for a large drainage lumen and the advancement of an endoscope through the stent lumen for direct necrosectomy has shifted paradigms in the management of WOPN by improving efficacy and decreasing complications associated with these procedures.
ENDOSCOPY VERSUS PERCUTAENOUS OR SURGERY DRAINAGE
A recent randomized multicenter trial from 2012 directly compared endoscopic necrosectomy and surgical necrosectomy (video-assisted retroperitoneal debridement with open laparoscopic necrosectomy for rescue) in 22 patients (121). Their results showed that endoscopic therapy was associated with a lower post- procedure inflammatory response (as demonstrated by interleukin levels), a lower complication rate, fewer pancreatic fistulae developments, and less pancreatic enzyme use on 6-month follow-up. A more recent from 2014 directly compared a step-up approach starting with percutaneous drainage and escalating to more invasive therapy as needed to direct endoscopic necrosectomy in 24 patients (122). Their results demonstrated a resolution rate of 92% versus 25% in the necrosectomy versus percutaneous drainage group, with 9 of 12 patients requiring surgery after percutaneous drainage alone. Additionally, less antibiotic use, pancreatic insufficiency, and hospitalization was seen in the endoscopic necrosectomy group.
ERCP FOR PANCREATIC DUCT EXPLORATION
An important component in the management of PFCs is ensuring the integrity of the pancreatic duct (PD) via ERCP. Disruptions in the PD are associated with an increased severity of pancreatitis, an increased risk of recurrent attacks of pancreatitis and long-term complications, and a decreased rate of PFC resolution after drainage (123-127).
PD DISRUTPION AND SEVERITY OF PANCREATITIS
A PD disruption has been shown to be associated with a more severe course of pancreatitis. A retrospective review of 105 patientswith acute pancreatitis found that nearly half of patients with severe pancreatitis had concurrent PD disruption, while a normal PD was noted in 100% of patients with mild pancreatitis (123). Similarly, in another review of 144 patients with severe pancreatitis, Lau et al found that patients with a PD leak were 3.4 times more likely to have pancreatic necrosis (127). Thus, assessing for a PD disruption in patients with pancreatitis is an important prognosticating step.
PD DISRUPTION AND RECURRENT PANCREATITIS / LONG-TERM COMPLICATIONS In addition to predicting the severity of pancreatitis, a PD disruption can also predict the likelihood of long-term complications and recurrent episodes of pancreatitis. Howard et al looked at 14 patients with WOPN who developed recurrent pancreatitis after initially-successful debridement and found that all 14 patients had a pancreatic duct abnormality on either ERCP or MRCP (124). No other predictive factor of recurrence was identified. Nealon et al demonstrated that in 174 patients with severe pancreatitis, long-term complications such as sepsis and recurrent pancreatitis occurred in 36-38% versus 0% and 62-89% versus 7% of patients with an abnormal PD compared to those with a normal PD (125).
PD DISRUPTION AND PFC RESOLUTION
Assessing for PD disruptions can also predict treatment success. In the same study as abovementioned, Nealon et al demonstrated that altered PD anatomy is directly correlated with a decreased rate of pseudocyst resolution (125). In 563 patients with pseudocysts, they found that spontaneous resolution occurred only in 0-5% of patients with a ductal disruption compared to 87% of patients with a normal pancreatic duct. Similarly, Trevino et al demonstrated improved PFC resolution in both pseudocysts and WOPN in patients who underwent PFC drainage with transpapillary PD stenting compared with PFC drainage alone (97.5% vs 80%) (126). Of note, undergoing ERCP was not associated with any increase in mortality, the need for necrosectomy, or hospital length of stay.
CONCLUSION
Pancreatitis can frequently result in the development of fluid collections, ranging from simple pseudocysts to WOPN. The initial step in management of these collections is ensuring adequate nutritional support is provided. Enteral nutrition is preferred over parenteral nutrition, with post-pancreatic jejunal feeding being the optimal enteral route in patients with moderate or severe disease. Endoscopic drainage can be successfully accomplished with improved safety and efficacy as compared to surgical or radiologic approaches. Furthermore, patients with WOPN can safely undergo endoscopic necrosectomy, obviating the need for surgical exploration. Lastly, ERCP with PD exploration should be concurrently performed to evaluate for evidence of PD disruption in all patients with PFCs. In summary, all forms of PFC can be safely and effectively managed by a variety of endoscopic procedures.
TAKE HOME MESSAGES
1. Classifying pancreatic fluid collections (PFCs) using the revised Atlanta criteria is critical for optimizing management and treatment.
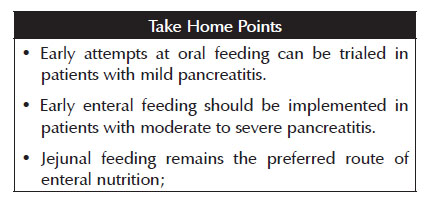
2. Early attempts at oral feeding can be trialed in patients with mild pancreatitis. Early enteral feeding should be implemented in patients with moderate to severe pancreatitis.
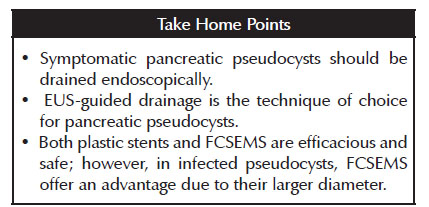
3. Symptomatic pancreatic pseudocysts should be drained endoscopically. EUS-guided drainage is the technique of choice. Both plastic stents and FCSEMS are efficacious and safe; however, in infected pseudocysts, FCSEMS offer an advantage due to their larger diameter.
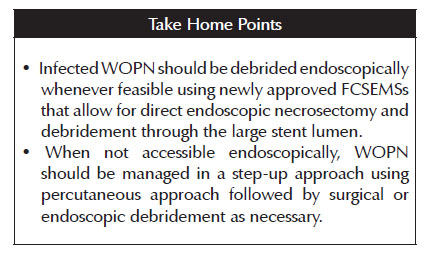
4. Infected WOPN should be debrided endoscopically whenever feasible using newly approved FCSEMSs that allow for direct endoscopic necrosectomy and debridement through the stent lumen. When not accessibleendoscopically, WOPN should be managed in a step-up approach using percutaneous approach followed by surgical or endoscopic debridement as necessary.
5. ERCP with PD exploration should be performed concurrent to PFC drainage. Any pancreatic ductal disruption should be managed with ERCP with intention to provide endoscopic stenting.
BIBLIOGRAPHIC REFERENCES
1. Poornachandra, K.S., et al., Clinical, biochemical, and radiologic parameters at admission predicting formation of a pseudocyst in acute pancreatitis. J Clin Gastroenterol. 2011;45(2):159-63. [ Links ]
2. Banks, P.A., M.L. Freeman, and G. Practice Parameters Committee of the American College of, Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379-400. [ Links ]
3. Takeda, K., et al., Pancreatic ischemia associated with vasospasm in the early phase of human acute necrotizing pancreatitis. Pancreas. 2005;30(1):40-9. [ Links ]
4. Wall, I., et al., Decreased mortality in acute pancreatitis related to early aggressive hydration. Pancreas. 2011;40(4):547-50. [ Links ]
5. Gardner, T.B., et al., Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol. 2008;6(10):1070-6. [ Links ]
6. Warndorf, M.G., et al., Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):705-9. [ Links ]
7. Baillargeon, J.D., et al., Hemoconcentration as an early risk factor for necrotizing pancreatitis. Am J Gastroenterol. 1998;93(11):2130-4. [ Links ]
8. Mounzer, R., et al., Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142(7):1476-82; quiz e15-6. [ Links ]
9. Tenner, S., Initial management of acute pancreatitis: critical issues during the first 72 hours. Am J Gastroenterol. 2004;99(12):2489-94. [ Links ]
10. Wu, B.U., et al., Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710-7. [ Links ]
11. Gardner, T.B., et al., Faster rate of initial fluid resuscitation in severe acute pancreatitis diminishes in-hospital mortality. Pancreatology. 2009;9(6):770-6. [ Links ]
12. Besselink, M.G., et al., Timing of surgical intervention in necrotizing pancreatitis. Arch Surg. 2007;142(12):1194-201. [ Links ]
13. Jiang, K., et al., Present and future of prophylactic antibiotics for severe acute pancreatitis. World J Gastroenterol. 2012;18(3):279-84. [ Links ]
14. Tenner, S., et al., American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-15; 1416. [ Links ]
15. Garg, P.K., et al., Primary conservative treatment results in mortality comparable to surgery in patients with infected pancreatic necrosis. Clin Gastroenterol Hepatol. 2010;8(12):1089-94. [ Links ]
16. Mouli, V.P., V. Sreenivas, and P.K. Garg, Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology. 2013;144(2):333-40. [ Links ]
17. 1Attasaranya, S., E.L. Fogel, and G.A. Lehman, Choledocholithiasis, ascending cholangitis, and gallstone pancreatitis. Med Clin North Am. 2008;92(4):925-60. [ Links ]
18. Acosta, J.M. and C.L. Ledesma, Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974;290(9):484-7. [ Links ]
19. Fogel, E.L. and S. Sherman, ERCP for gallstone pancreatitis. N Engl J Med. 2014;370(2):150-7. [ Links ]
20. Tse, F. and Y. Yuan, Early routine endoscopic retrograde cholangiopancreatography strategy versus early conservative management strategy in acute gallstone pancreatitis. Cochrane Database Syst Rev. 2012;5:CD009779. [ Links ]
21. Fan, S.T., et al., Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328(4):228-32. [ Links ]
22. Folsch, U.R., et al., Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med. 1997;336(4):237-42. [ Links ]
23. Neoptolemos, J.P., et al., Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2(8618):979-83. [ Links ]
24. Oria, A., et al., Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction: a randomized clinical trial. Ann Surg. 2007;245(1):10-7. [ Links ]
25. Zhou, M.Q., N.P. Li, and R.D. Lu, Duodenoscopy in treatment of acute gallstone pancreatitis. Hepatobiliary Pancreat Dis Int. 2002;1(4):608-10. [ Links ]
26. Shrode, C.W. and M. Kahaleh, Early ERCP in acute gallstone pancreatitis without cholangitis: a need for systematic biliary sphincterotomy! JOP. 2009;10(6): 701-2. [ Links ]
27. Bradley, E.L., 3rd, A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128(5): 586-90. [ Links ]
28. Banks, P.A., et al., Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-11. [ Links ]
29. Goodgame, J.T. and J.E. Fischer, Parenteral nutrition in the treatment of acute pancreatitis: effect on complications and mortality. Ann Surg. 1977;186(5):651-8. [ Links ]
30. Al-Omran, M., et al., Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;(1):CD002837. [ Links ]
31. Olah, A. and L. Romics, Jr., Enteral nutrition in acute pancreatitis: a review of the current evidence. World J Gastroenterol. 2014;20(43):16123-31. [ Links ]
32. Corcoy R, S.J., Domingo P et al. , Nutrition in patients with severe acute pancreatitis. Nutrition. 1988;4:269-75. [ Links ]
33. Mutch, K.L., et al., Cost-analysis of nutrition support in patients with severe acute pancreatitis. Int J Health Care Qual Assur. 2011;24(7):540-7. [ Links ]
34. Li, J.Y., et al., Enteral nutrition within 48 hours of admission improves clinical outcomes of acute pancreatitis by reducing complications: a meta-analysis. PLoS One. 2013;8(6):e64926. [ Links ]
35. Chang, Y.S., et al., Nasogastric or nasojejunal feeding in predicted severe acute pancreatitis: a meta-analysis. Crit Care. 2013;17(3):R118. [ Links ]
36. Singh, N., et al., Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis: a noninferiority randomized controlled trial. Pancreas. 2012;41(1):153-9. [ Links ]
37. Makola, D., et al., Efficacy of enteral nutrition for the treatment of pancreatitis using standard enteral formula. Am J Gastroenterol. 2006;101(10):2347-55. [ Links ]
38. Windsor, A.C., et al., Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42(3):431-5. [ Links ]
39. Parks, R.W., et al., Management of pancreatic pseudocysts. Ann R Coll Surg Engl. 2000;82(6):383-7. [ Links ]
40. Johnson, M.D., et al., Surgical versus nonsurgical management of pancreatic pseudocysts. J Clin Gastroenterol. 2009;43(6):586-90. [ Links ]
41. Melman, L., et al., Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg Endosc. 2009;23(2):267-71. [ Links ]
42. Varadarajulu, S., et al., Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145(3):583-90. [ Links ]
43. Heider, R., et al., Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg. 1999;229(6):781-7. [ Links ]
44. Spivak, H., et al., Management of pancreatic pseudocysts. J Am Coll Surg. 1998;186(5):507-11. [ Links ]
45. Lang, E.K., R.M. Paolini, and A. Pottmeyer, The efficacy of palliative and definitive percutaneous versus surgical drainage of pancreatic abscesses and pseudocysts: a prospective study of 85 patients. South Med J. 1991;84(1):55-64. [ Links ]
46. Adams, D.B. and M.C. Anderson, Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg. 1992;215(6):571-6. [ Links ]
47. Akshintala, V.S., et al., A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc. 2014;79(6):921-8. [ Links ]
48. Kahaleh, M., et al., Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38(4):355-9. [ Links ]
49. Rogers, B.H., N.J. Cicurel, and R.W. Seed, Transgastric needle aspiration of pancreatic pseudocyst through an endoscope. Gastrointest Endosc. 1975;21(3):133-4. [ Links ]
50. Kozarek, R.A., et al., Endoscopic drainage of pancreatic pseudocysts. Gastrointest Endosc. 1985;31(5):322-7. [ Links ]
51. Cremer, M. and J. Deviere, Endoscopic management of pancreatic cysts and pseudocysts. Gastrointest Endosc. 1986;32(5):367-8. [ Links ]
52. Cremer, M., J. Deviere, and L. Engelholm, Endoscopic management of cysts and pseudocysts in chronic pancreatitis: long-term follow-up after 7 years of experience. Gastrointest Endosc. 1989;35(1):1-9. [ Links ]
53. Binmoeller, K.F., et al., Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 1995;42(3):219-24. [ Links ]
54. Baron, T.H., et al., Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56(1):7-17. [ Links ]
55. Beckingham, I.J., et al., Long term outcome of endoscopic drainage of pancreatic pseudocysts. Am J Gastroenterol. 1999;94(1):71-4. [ Links ]
56. Cahen, D., et al., Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37(10):977-83. [ Links ]
57. De Palma, G.D., et al., Endoscopic drainage of pancreatic pseudocysts: a long-term follow-up study of 49 patients. Hepatogastroenterology. 2002;49(46):1113-5. [ Links ]
58. Hookey, L.C., et al., Endoscopic drainage of pancreatic- fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63(4):635-43. [ Links ]
59. Sharma, S.S., N. Bhargawa, and A. Govil, Endoscopic management of pancreatic pseudocyst: a long-term follow- up. Endoscopy. 2002;34(3):203-7. [ Links ]
60. Smits, M.E., et al., The efficacy of endoscopic treatment of pancreatic pseudocysts. Gastrointest Endosc. 1995;42(3):202-7. [ Links ]
61. Weckman, L., et al., Endoscopic treatment of pancreatic pseudocysts. Surg Endosc. 2006;20(4):603-7. [ Links ]
62. Sanchez Cortes, E., et al., Endoscopic cystenterostomy of nonbulging pancreatic fluid collections. Gastrointest Endosc. 2002;56(3):380-6. [ Links ]
63. Antillon, M.R., et al., Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63(6):797-803. [ Links ]
64. Grimm, H., K.F. Binmoeller, and N. Soehendra, Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest Endosc. 1992;38(2):170-1. [ Links ]
65. Wiersema, M.J., Endosonography-guided cystoduodenostomy with a therapeutic ultrasound endoscope. Gastrointest Endosc. 1996;44(5):614-7. [ Links ]
66. Giovannini, M., et al., Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33(6):473-7. [ Links ]
67. Azar, R.R., et al., Wire-guided pancreatic pseudocyst drainage by using a modified needle knife and therapeutic echoendoscope. Gastrointest Endosc. 2006;63(4):688-92. [ Links ]
68. Barthet, M., et al., Clinical usefulness of a treatment algorithm for pancreatic pseudocysts. Gastrointest Endosc. 2008;67(2):245-52. [ Links ]
69. Lopes, C.V., et al., Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42(4):524-9. [ Links ]
70. Varadarajulu, S., et al., Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc. 2007;66(6):1107-19. [ Links ]
71. Sadik, R., et al., EUS-guided drainage is more successful in pancreatic pseudocysts compared with abscesses. World J Gastroenterol. 2011;17(4):499-505. [ Links ]
72. Varadarajulu, S., et al., Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15(11):2080-8. [ Links ]
73. Varadarajulu, S., et al., Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68(6):1102-11. [ Links ]
74. Park, D.H., et al., Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41(10):842-8. [ Links ]
75. Penn, D.E., et al., Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 2012;76(3):679-84. [ Links ]
76. Weilert, F., et al., Endoscopic ultrasound-guided drainage of pancreatic fluid collections with indeterminate adherence using temporary covered metal stents. Endoscopy. 2012;44(8):780-3. [ Links ]
77. Fabbri, C., et al., Endoscopic ultrasound-guided drainage of pancreatic fluid collections. World J Gastrointest Endosc. 2012;4(11):479-88. [ Links ]
78. Tarantino, I., et al., EUS-guided self-expandable stent placement in 1 step: a new method to treat pancreatic abscess. Gastrointest Endosc. 2009;69(7):1401-3. [ Links ]
79. Talreja, J.P., et al., Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video). Gastrointest Endosc. 2008;68(6):1199-203. [ Links ]
80. Berzosa, M., et al., Single-step endoscopic ultrasonography- guided drainage of peripancreatic fluid collections with a single self-expandable metal stent and standard linear echoendoscope. Endoscopy. 2012;44(5):543-7. [ Links ]
81. Navaneethan, U., 734: Endoscopic Transmural Drainage of Pancreatic Pseudocysts: Multiple Plastic Stents Versus Metal Stents - A Systematic Review and Meta-Analysis. Gastrointest Endosc. 2014;79(5):167-8. [ Links ]
82. Sharaiha, R.Z., et al., Metal versus plastic for pancreatic pseudocyst drainage: clinical outcomes and success. Gastrointest Endosc. 2015. [ Links ]
83. Itoi, T., et al., Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc. 2012;75(4):870-6. [ Links ]
84. Shah, R.J., et al., Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015;13(4):747-52. [ Links ]
85. Gornals, J.B., et al., Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc. 2013;27(4):1428-34. [ Links ]
86. Walter, D., et al., A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a prospective cohort study. Endoscopy. 2015;47(1):63-7. [ Links ]
87. Aranda-Narvaez, J.M., et al., Acute necrotizing pancreatitis: Surgical indications and technical procedures. World J Clin Cases. 2014;2(12):840-5. [ Links ]
88. Freeman, M.L., et al., Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176-94. [ Links ]
89. Karakayali, F.Y., Surgical and interventional management of complications caused by acute pancreatitis. World J Gastroenterol. 2014;20(37):13412-23. [ Links ]
90. Bradley, E.L., 3rd, Management of infected pancreatic necrosis by open drainage. Ann Surg. 1987;206(4):542-50. [ Links ]
91. Sarr, M.G., et al., Acute necrotizing pancreatitis: management by planned, staged pancreatic necrosectomy/debridement and delayed primary wound closure over drains. Br J Surg. 1991;78(5):576-81. [ Links ]
92. Fernandez-del Castillo, C., et al., Debridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998;228(5):676-84. [ Links ]
93. Wittau, M., et al., Changing role of surgery in necrotizing pancreatitis: a single-center experience. Hepatogastroenterology. 2010;57(102-103):1300-4. [ Links ]
94. Rodriguez, J.R., et al., Debridement and closed packing for sterile or infected necrotizing pancreatitis: insights into indications and outcomes in 167 patients. Ann Surg. 2008;247(2):294-9. [ Links ]
95. Raraty, M.G., et al., Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg. 2010;251(5):787-93. [ Links ]
96. Parikh, P.Y., et al., Pancreatic necrosectomy: North American mortality is much lower than expected. J Am Coll Surg. 2009;209(6):712-9. [ Links ]
97. Howard, T.J., et al., Declining morbidity and mortality rates in the surgical management of pancreatic necrosis. J Gastrointest Surg. 2007;11(1):43-9. [ Links ]
98. van Santvoort, H.C., et al., A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16): 1491-502. [ Links ]
99. Wronski, M., et al., Minimally invasive treatment of infected pancreatic necrosis. Prz Gastroenterol. 2014;9(6):317-24. [ Links ]
100. Gambiez, L.P., et al., Retroperitoneal approach and endoscopic management of peripancreatic necrosis collections. Arch Surg. 1998;133(1):66-72. [ Links ]
101. van Santvoort, H.C., et al., Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg. 2007;31(8):1635-42. [ Links ]
102. Freeny, P.C., et al., Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 1998;170(4):969-75. [ Links ]
103. van Baal, M.C., et al., Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2011;98(1):18-27. [ Links ]
104. Lee, J.K., et al., The efficacy of nonsurgical treatment of infected pancreatic necrosis. Pancreas. 2007;34(4):399-404. [ Links ]
105. Chang, Y.C., et al., No debridement is necessary for symptomatic or infected acute necrotizing pancreatitis: delayed, mini-retroperitoneal drainage for acute necrotizing pancreatitis without debridement and irrigation. Dig Dis Sci. 2006;51(8):1388-95. [ Links ]
106. Bruennler, T., et al., Outcome of patients with acute, necrotizing pancreatitis requiring drainage-does drainage size matter? World J Gastroenterol. 2008;14(5):725-30. [ Links ]
107. Mortele, K.J., et al., CT-guided percutaneous catheter drainage of acute necrotizing pancreatitis: clinical experience and observations in patients with sterile and infected necrosis. AJR Am J Roentgenol. 2009;192(1):110-6. [ Links ]
108. Besselink, M.G., et al., Minimally invasive 'step-up approach' versus maximal necrosectomy in patients with acute necrotising pancreatitis (PANTER trial): design and rationale of a randomised controlled multicenter trial [ISRCTN13975868]. BMC Surg. 2006;6:6. [ Links ]
109. Baron, T.H., et al., Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111(3):755-64. [ Links ]
110. Papachristou, G.I., et al., Peroral endoscopic drainage/ debridement of walled-off pancreatic necrosis. Ann Surg. 2007;245(6):943-51. [ Links ]
111. Seewald, S., et al., Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm (videos). Gastrointest Endosc. 2005;62(1):92-100. [ Links ]
112. Voermans, R.P., et al., Endoscopic transmural debridement of symptomatic organized pancreatic necrosis (with videos). Gastrointest Endosc. 2007;66(5):909-16. [ Links ]
113. Escourrou, J., et al., Peroral transgastric/transduodenal necrosectomy: success in the treatment of infected pancreatic necrosis. Ann Surg. 2008;248(6):1074-80. [ Links ]
114. Charnley, R.M., et al., Endoscopic necrosectomy as primary therapy in the management of infected pancreatic necrosis. Endoscopy. 2006;38(9):925-8. [ Links ]
115. Seifert, H., et al., Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009;58(9):1260-6. [ Links ]
116. Gardner, T.B., et al., Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011;73(4):718-26. [ Links ]
117. Fabbri, C., et al., Endoscopic ultrasound-guided transmural drainage of infected pancreatic fluid collections with placement of covered self-expanding metal stents: a case series. Endoscopy. 2012;44(4):429-33. [ Links ]
118. Antillon, M.R., et al., Transgastric endoscopic necrosectomy with temporary metallic esophageal stent placement for the treatment of infected pancreatic necrosis (with video). Gastrointest Endosc. 2009;69(1):178-80. [ Links ]
119. Sarkaria, S., et al., Pancreatic necrosectomy using covered esophageal stents: a novel approach. J Clin Gastroenterol. 2014;48(2):145-52. [ Links ]
120. Attam, R., et al., Endoscopic transluminal drainage and necrosectomy by using a novel, through-the-scope, fully covered, large-bore esophageal metal stent: preliminary experience in 10 patients. Gastrointest Endosc. 2014;80(2):312-8. [ Links ]
121. Bakker, O.J., et al., Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307(10):1053-61. [ Links ]
122. Kumar, N., D.L. Conwell, and C.C. Thompson, Direct endoscopic necrosectomy versus step-up approach for walled-off pancreatic necrosis: comparison of clinical outcome and health care utilization. Pancreas. 2014;43(8):1334-9. [ Links ]
123. Neoptolemos, J.P., N.J. London, and D.L. Carr-Locke, Assessment of main pancreatic duct integrity by endoscopic retrograde pancreatography in patients with acute pancreatitis. Br J Surg. 1993;80(1):94-9. [ Links ]
124. Howard, T.J., et al., Pancreatic duct strictures are a common cause of recurrent pancreatitis after successful management of pancreatic necrosis. Surgery. 2004;136(4):909-16. [ Links ]
125. Nealon, W.H., et al., A unifying concept: pancreatic ductal anatomy both predicts and determines the major complications resulting from pancreatitis. J Am Coll Surg. 2009;208(5):790-9; [ Links ].
126. Trevino, J.M., A. Tamhane, and S. Varadarajulu, Successful stenting in ductal disruption favorably impacts treatment outcomes in patients undergoing transmural drainage of peripancreatic fluid collections. J Gastroenterol Hepatol. 2010;25(3):526-31. [ Links ]
127. Lau, S.T., et al., A pancreatic ductal leak should be sought to direct treatment in patients with acute pancreatitis. Am J Surg. 2001;181(5):411-5. [ Links ]
Correspondence:
Michel Kahaleh
E-mail: mkahaleh@gmail.com
Recibido: 12-11-2017
Aceptado: 11-04-2018