Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Gastroenterología del Perú
versão impressa ISSN 1022-5129
Rev. gastroenterol. Perú vol.39 no.2 Lima abr./jun. 2019
REPORTE DE CASO
Rectus abdominis muscle flap for repair of the gastrojejunal leakage after distal gastrectomy with Billroth II anastomosis for gastric cancer
Colgajo del músculo recto abdominal para reparación de la dehiscencia gastroyeyunal luego de gastrectomía distal con anastomosis Billroth II por cáncer gástrico
Juan A. Díaz-Plasencia1,2, Carlos R. Guzmán-Gavidia1,2, Hugo D. Valencia- Mariñas1,2, Edgar F. Yan-Quiroz2,3, Melisa S. Díaz-Villazon2
1 Department of Abdomen, Regional Institute of Neoplastic Diseases "Luis Pinillos Ganoza". Trujillo, Peru.
2 Private University Antenor Orrego. Trujillo, Peru.
3 Oncologic Surgery Service, Hospital of High Complexity "Virgen de la Puerta". Trujillo, Peru.
ABSTRACT
Anastomotic leakages at the gastrojejunostomy site are difficult to repair, due to complex gastrointestinal anatomy. This is the first study reporting clinical use of rectus abdominis muscle (RAM) flap for repair of gastrojejunostomy leakage. A patient with leakage of gastrojejunostomy after distal gastrectomy with Billrroth II anastomosis for gastric cancer underwent repair using left RAM flap, based on superior epigastric artery. Rectus abdominis muscle flap, after being harvested was then anchored to the edges of the leak of gastrojejunostomy with few interrupted 2-0 vicryl sutures. Gastrojejunostomy leak sealed in the two cases. Rectus abdominis muscle flap for closure of gastrointestinal defect is a simple, technically easy and dependable procedure, which can be performed, quickly in critically ill patients. It can be used for repair of a large gastrointestinal defect with friable edges when omentum is not available or when other conventional methods are impractical.
Keywords: Jejunostomy; Rectus abdominis; Gastrectomy; Gastric cancer (source: MeSH NLM).
RESUMEN
Las dehiscencias anastomóticas en el sitio de gastroyeyunostomía son difíciles de reparar, debido a la compleja anatomía gastrointestinal. Este es el primer estudio que comunica el uso clínico del colgajo del músculo recto abdominal (MRA) para la reparación de la dehiscencia de gastroyeyunostomía. A un paciente con dehiscencia de gastroyeyunostomía, luego de una gastrectomía distal con anastomosis Billrroth II para cáncer gástrico, se le realizó una reparación utilizando colgajo izquierdo del MRA, basado en la arteria epigástrica superior. El colgajo del músculo recto abdominal, después de ser extraído, se fijó a los bordes de la dehiscencia de la gastroyeyunostomía con pocas suturas de vicryl 2-0 interrumpidas. La dehiscencia de la gastroyeyunostomía fue sellada. El colgajo del músculo reto abdominal para el cierre del defecto gastrointestinal es un procedimiento simple, técnicamente fácil y confiable, que puede realizarse rápidamente en pacientes críticamente enfermos. Se puede utilizar para la reparación de un gran defecto gastrointestinal con bordes friables cuando el omento no está disponible o cuando otros métodos convencionales no son prácticos.
Palabras clave: Yeyunostomía; Recto abdominal; Gastrectomía; Cáncer gástrico (fuente: DeCS BIREME).
INTRODUCTION
The rectus abdominis, as a flap-like muscle, is an important postural muscle that plays a role in movement and in stabilization of the trunk. It helps the lower ribs, especially during expiration. It compresses the abdomen, supports the viscera, and is very important in weight lifting and other sports. The rectus abdominis muscle (RAM), as a myocutaneous flap, has been used in numerous surgical reconstructions: in the reconstruction of scrotum (1), reconstruction of the breast (2,3), closure of sternal defects (4), in head and neck reconstructive surgery (5), in the treatment of enterocutaneous fistula (6,7), in the correction of pharyngoesophageal defects (8), in soft tissue reconstruction of the hand (9), in treatment of complex urethrovaginal fistulae (10,11), in the repair of difficult vesicovaginal fistulae (12), in vaginal reconstruction (13), in the reconstruction of full-thickness thoracic wall defects (14), in the reconstruction of ankle and foot defects (15), and in abdominoperineal resection after radiotherapy (16). Additionally, Agarwal et al. (17) successfully used right RAM flap for surgical closure of duodenal fistulae in six patients; and Chander et al. (18) treated six patients with post-surgical duodenal fistulas with this technique and the leak was completely sealed in all patients.
For resectable carcinoma of the antropyloric region, gastrojejunostomyis performed after radical subtotal gastrectomy to maintain continuity of the gastrointestinal (GI) tract and this procedure can be done by either an open or a laparoscopic approach. Leakage from the anastomosis usually occurs around postoperative day 4-7 and is associated with high morbidity and mortality and it requires reexploration, drainage, and a feeding jejunostomy (19). Anastomotic leakage in lean patients undergoing nonbariatric surgery commonly presents with tachycardia, fever, physical signs of peritonitis, reduced urine output, respiratory insufficiency, or elevated white blood cell count. Any of these findings prompt the prudent surgeon to consider the diagnosis of leakage from a gastrointestinal anastomosis (20).
The causal trilogy of the anastomotic leakage includes to a poor blood supply, an excessive tension of the tissues and an unequal coaptacion of the anastomotic edges, being on the first of these factors where the rectus abdominis muscle flap exerts its main effect. The approach of the anastomotic dehiscences should be appropriate and timely in order to prevent further unnecessary morbidity and mortality with inadequate maneuvers (21,22) so we presented a case with partial dehiscence of the gastrojejunal anastomosis (GJA) after Billroth II subtotal gastrectomy for antro-pyloric gastric cancer who underwent this flap RAM in order to expand its indications for these cases.
CASE REPORT
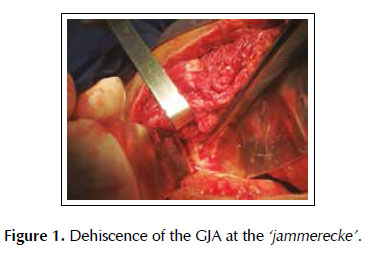
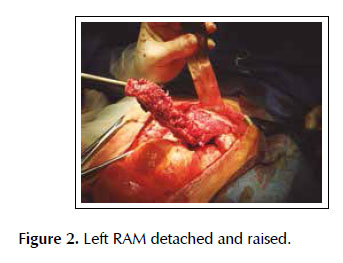
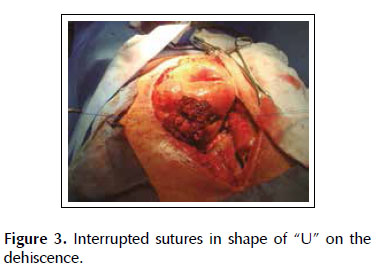
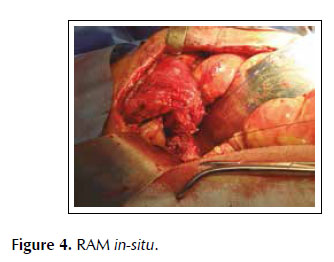
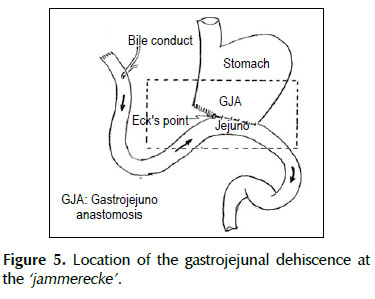
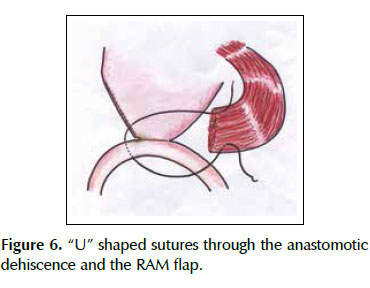
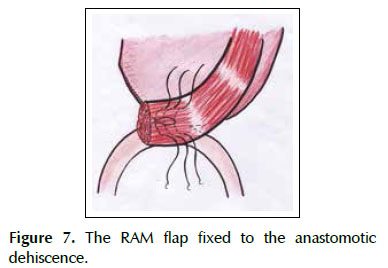
A 53-years old female patient with a history of weight loss (about 15 kg in 6 months), nausea, vomiting and epigastric pain who presents at the clinical exam umbilical pain and epigastric mass. An abdominal computed tomography image revealed a mural thickening of the wall of the antro-pyloric region of the stomach and an endoscopic biopsy of this area showed poorly differentiated signet ring adenocarcinoma. The patient underwent a distal subtotal gastrectomy and Billroth II gastrojejunal-anastomosis with a Hofmeister-Finsterer reconstruction and D2 lymphadenectomy. Evolves without complications and on the 8th postoperative day presents fever, diarrhoea, abdominal distention and peritoneal reaction on physical examination. She underwent exploratory laparotomy and a partial dehiscence of approximately 2 cm in diameter at the ‘jammerecke’of the gastrojejunal anastomosis was observed. This partial dehiscence was closured with a RAM flap (Figures 1, 2, 3 and 4). During three days developed minor leak (output < 200 ml/day) for the peritoneal drainage. Afterwards, her postoperative evolution was uneventful and was discharged on the 17th postoperative day.
Surgical technique
We describe a novel technique for repair of the postsurgical external leak of the gastrojejunostomy with a rectus abdominis muscle flap. Informed consent was taken and patient was re-explored through the same incision. It proved a partial dehiscence of the GJAat the ‘jammerecke’, we proceeded to the dissection of the rectus abdominis muscle taking care to preserve its proximal vascular supply. Left RAM was separated from anterior and posterior rectus sheath and laterally and mobilized from the rectus sheath and then detached from lower end where the deep inferior epigastric artery was ligated. The flap was then raised and sutured to the gastrojejunal dehiscence with three 2-0 vicrylinterrupted tension free suture manner of "U" shaped sutures. The suturing needle enters on the outer surface of the muscle, includes the total thickness of the muscle flap and the viable edges of the dehiscence and penetrates from the inner to the outer surface of muscle on the opposite side, and the vicryl suture is gently tied on the outer surface of the flap avoiding excessive tension (Figures 5, 6 and 7). We instilled a diluted methylene blue solution via nasogastric tube to check water tightness of the suture closure.
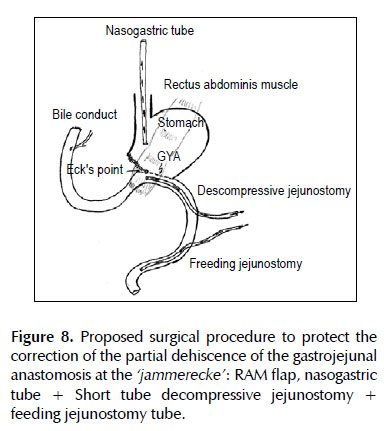
Two standard Witzel-type tube jejunostomy were added and both tubes brought out onto the left flank. Aproximal short tube descompressive jejunostomy (a 18 Fr diameter tube) 20 cm from the Treitz ligament on the side opposing the jejunal mesenterium for a permanent active suction was kept under the gastrojejunostomy which allow it to maintain a low intraluminal pressure in this area and may allow time for the gastrojejunal leakage to heal. A distal feeding jejunostomy tube (a 20 Fr diameter tube) 40 cm from the Treitz ligament was performed (Figure 8). Because of a higher degree of peritoneal contamination in this case, abdomen was left open as a laparostomy. The patient rec8eived ventilatory support, broad - spectrum antibiotic therapy to control the associated infection/septicemia.
DISCUSSION
The dehiscence of the gastrojejunostomy (GJA) after Billroth II distal subtotal gastrectomy with Hofmeister-Finsterer reconstruction mostly occurs at the‘Jammerecke’ of the Billroth II procedure. We defined this area to the junction of the gastrojejunostomy to the lesser curvature of the stomach just on the right side of the GJA where the anastomotic tensile strength is greater. Additionally, at this point we routinely placed a seromuscular suturetaking seromuscular bites of both sides from the jejunum to the gastric stump with the aim to reduce the tensile strength, but this could cause local ischemia and ultimately lead to a bad healing. Billroth’s first gastric resection was a gastroduodenal anastomosis with partial closure of the aboral gastric segment. Observance of leaks at the lesser curvature in the anastomosis led to its being called the ‘jammerecke’ (‘crying corner’ or ‘angle of sorrow’), a technical matter that has long since been overcome by surgeons (23). The so-called ‘jammerecke’ is traditionally covered by a triple seromuscular suture, including the front wall of the stomach, the small intestine, and the back wall of the stomach (24,25).
The dehiscence of the GJA usually occurs from the fifth to the seventh day of the surgery and the clinical picture corresponds to a secondary postoperative peritonitis with systemic inflammatory response subsequent, severe sepsis, septic shock and multiple organ failure if the leak of the anastomosis is not corrected early. Anastomotic disruption after distal gastrectomy is perhaps the most dreaded complication after gastrointestinal surgery (26), with a total incidence rate ranging from 2.2% to 6.8% and a mortality rate between 26.3% and 33% (27,28). Tamanes et al. (29) observed only one case of dehiscence of the gastrojejunal anastomosis after 726 gastrectomies, representing a percentage of 0.13%, although lately, Oh et al. (30) reported a 4.2% leak rate after gastrectomy for gastric cancer. At our institution, 130 subtotal gastrectomies were performed between 2008 and 2015, and there were two partial dehiscences of GJA, all of which constitutes a 2.4%, which is similar to the 1,3% leak rate published by Pickleman et al. (21) based on previous studies (17,31), if the patient has an acute generalized peritonitis and the dehiscence has not fistulized, it proceeds to the immediate reoperation, and a broader gastrectomy with gastrojejunostomy, in a healthy tissue may be necessary. The simple suture of dehiscence usually is not enough and it is only advisable in minimum dehiscences; and a descompressive gastrostomy may be useful to protect the new suture. A direct surgical approach to the leakage is always dangerous, because of the poor status of patient and of the local state of tissues, with a higher risk of aggravating the injury and carry out an aggressive intervention, even a total gastrectomy, performed in a septic environment, unfavorable for making surgical maneuvers and sutures more secure (29). The procedures involve disassembling the GJA and make a Roux-en-Y procedure or complete gastrectomy with esophagojejunal anastomosis involving higher risk of morbidity and mortality rates in a septic critically ill patient. These alternatives could be indicated when dehiscences are more extensive.
In the subtotal gastrectomy for cancer, the greater and lesser omentectomy is part of the surgical procedure, so when surgeons dealing with a partial dehiscence of the GJA, they can not make use of the greater omentum as a Graham’s patch that could be effective in controlling the leak. Therefore, in redo cases where omentum is not available or the cases where repair with omentum fails, Agarwal et al. (17) have used to rectus abdominis muscle (RAM) flap to repair of duodenal fistula.
In our experience, we used the RAM flap to cover the defect after removal of devitalized tissue and subsequent coverage of the defect associated with nasogastric decompression and antibiotic therapy of mixed coverage which controlled leakage through the anastomotic dehiscence. We believe that these measures, together with the open abdomen procedure, technique of planned staged relaparotomies with repeated lavage, enteral nutrition through jejunostomy, jejunostomy of decompression in the area of GJA and postoperative management in an intensive care unit are the pillars to reduce mortality in this abdominal disaster. In this study, our patient had poor nutritional status. In addition, the patient had generalized peritonitis and their peritoneum and bowel were severely inflamed. Despite the presence of poor prognostic factors, the RAM flap was successful in closing the fistula in this case. Our patient developed minor leak (output < 200 ml/day), which healed on conservative treatment, probably significant bulk of muscle ensured that leaking fistula remained low output and inflammatory adhesions between gastrojejunal dehiscense and the muscle flap prevented breakdown at the fistulous site. The patient in our series underwent laparostomy, which ensures drainage of residual sepsis; allows daily inspection of the status of flap and helps in early detection of gastrointestinal leak. We routinely added feeding jejunostomy in our patient; as it shortens the time to resumption of feeds and is a useful adjunct to repair of a difficult fistula.
Rectus abdominis has a constant and reliable vascular pedicle, making its use a popular and versatile flap. As the pedicle of superiorily based flap enters high up in the abdomen, there is no risk of bowel loop twisting around this flap and causing obstruction; inferiorly based flap is avoided for this reason. Success depends upon maintaining the adequate arterial supply and venous drainage of muscle so it can withstand the digestive properties of the gastroduodenal contents for a period long enough to permit healing (32).
Conventional wisdom dictates that healthy vascularized tissue should be incorporated in the repair of any defect with tissue loss or with friable edges. There are many advantages of this technique: Harvesting of only muscle flap is easier, there is no additional weakness of anterior abdominal wall because anterior and posterior rectus sheaths remain intact, and hence risk of incisional hernia is minimized. The rectus abdominis muscle provides better watertight closure and the use of a vascularized flap augments the blood supply to the previously devitalized area with an underlying severe inflammation associated to a dehiscence, thereby hastening the healing process and promoting tissue phagocytic activity on the inflammatory bed, all of which helps the local control of bacterial growth and counters the infection in much better way. It is assumed that myoglobin released from muscle is also a contributory factor in wound healing (7,31,33-36). It is known that the submucosa has a rich vascular and lymphatic plexus and it is a collagen rich layer which promotes better healing (37). Experimental work has shown that reepithelialization occurs over the muscle from the edges of defect and metaplastic adaptation of epithelium in to neomucosa takes place by 8-12 weeks (38,39).
However, the major disadvantage of this technique is the conspicuous abdominal scar (13). While the rectus abdominis muscle following intramuscular dissection of the deep inferior epigastric artery perforators after harvesting of the deep inferior epigastric perforator flap provides the well-known advantage of use of the lower abdominal tissue with preservation of the integrity of the abdominal wall musculature, postoperative problems such as abdominal asymmetry, bulges and reduced flexion capacity have been found, and these changes may be due to rectus abdominis muscle damage from ischemia or denervation (40). We observed an increased muscle thickness which was attributed to postoperative oedema that resolved with time. Lee et al. (40) used ultrasonography to assess the changes in rectus abdominis muscle thickness and contractility, preoperatively, 1-month and 1-year postoperatively, and demonstrated that all muscles retained contractility showing no evidence of muscle denervation and that intramuscular dissection of perforator vessels in the flap leads to minimal changes in the local morphology and contractility of the rectus abdominis muscle.
Erni and Harder (41) used in 20 consecutive patients the dissection of the rectus abdominis myocutaneous flap with complete preservation of the anterior rectus sheath: Nine patients underwent free TRAM flap transfers for breast reconstruction (10 flaps), and 11 patients underwent thoracic-wall reconstruction with a superiorly based pedicled flap and no abdominal bulging or hernia occurred in these patients. They suggested that the technical modification may enable the surgeon to dissect a rectus abdominis myocutaneous flap with maximal perforator-related flap perfusion and minimal donor-site morbidity. However, others did not report complications of the abdominal wall (16).
It should be performed a vertical incision in the abdominal wall in order to preserve the anterior rectus abdominis muscle and then use it if an anastomotic leaks occurs after distal gastrectomy with anastomotic partial dehiscence. We recommend a rectus abdominis muscle (RAM) flap for the management of postsurgical external gastrojejunal leaks as an alternative to other surgical techniques since is safe, simple, and feasible technique, it may provide local support to the salvage repair of gastrojejunal dehiscence until it reaches good healing. However, it is worth mentioning that this surgical technique is an extreme resource as long as there is no more experience.
Conflict of interest: None of the authors have any financial conflicts of interest.
REFERENCES
1. Young WA, Wright JK. Scrotal reconstruction with a rectus abdominis muscle flap. Br J Plastic Surg. 1988;41(2):190-3. [ Links ]
2. Robbins TH. Rectus abdominis myocutaneous flap for breast reconstruction. Aust N Z J Surg. 1979;49(5):527-30. [ Links ]
3. Arnez ZM, Smith RW, Eder E, Eder E, Solinc M, Kersnic M. Breast reconstruction by the free lower transverse rectus abdominis musculocutaneous flap. Br J Plast Surg. 1988;41(5):500-5. [ Links ]
4. Fernando B, Muszynski C, Mustoe T. Closure of a sternal defect with the rectus abdominis muscle after sacrifice of both internal mammary arteries. Ann Plastic Surg. 1988;21(5):468-71. [ Links ]
5. Urken ML, Turk JB, Weinberg H, Vickery C, Biller HF. The rectus abdominis free flap in head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 1991;117(8):857-66. [ Links ]
6. Carey JN, Sheckter CC, Watt AJ, Lee GK. Intra-abdominal pedicled rectus abdominis muscle flap for treatment of highoutput enterocutaneous fistulae: case reports and review of literature. J Plast Reconstr Aesthet Surg. 2013;66(8):1145-8. [ Links ]
7. Chang P, Chun JT, Bell JL. Complex enterocutaneous fistula: closure with rectus abdominis muscle flap. South Med J. 2000;93(6):599-602. [ Links ]
8. Jinming Z, Xiaoxuan C, Jieren P, Shujuan P. The rectus abdominis musculoperitoneal (RAMP) flap for the reconstruction of complicated pharyngoesophageal defects. Br J Plastic Surg. 2005;58(5):608-13. [ Links ]
9. Press BHJ, Chiu DT, Cunningham BL. The rectus abdominis muscle in difficult problems of hand soft tissue reconstruction. Br J Plastic Surg. 1990;43(4):419-25. [ Links ]
10. Bruce RG, El-Galley RE, Galloway N. Use of rectus abdominis muscle flap for the treatment of complex and refractory urethrovaginal fistulas. J Urology. 2000;163(4):1212-5. [ Links ]
11. Atan A, Tuncel A, Aslan Y. Treatment of refractory urethrovaginal fistula using rectus abdominis muscle flap in a six-year-old girl. Urology. 2007;69(2):384.e11-3. [ Links ]
12. Menchaca A, Akhyat M, Gleicher N, Gottlieb L, Bernstein J. The rectus abdominis muscle flap in a combined abdominovaginal repair of difficult vesicovaginal fistulae. A report of three cases. J Reprod Med. 1990;35(5):565-8. [ Links ]
13. Weiwei L, Zhifei L, Ang Z, Lin Z, Dan L, Qun Q. Vaginal reconstruction with the muscle-sparing vertical rectus abdominis myocutaneous flap. J Plast Reconstr Aesthet Surg. 2009;62(3):335-40. [ Links ]
14. Galli A, Raposio E, Santi P. Reconstruction of full-thickness defects of the thoracic wall by myocutaneous flap transfer: latissimus dorsi compared with transverse rectus abdominis. Scand J Plast Reconstr Surg Hand Surg. 1995;2(1)9:39-43. [ Links ]
15. Reath DB, Taylor JW. The segmental rectus abdominis free flap for ankle and foot reconstruction. Plastic Reconstruct Surg. 1991;88(5):824-8. [ Links ]
16. Casal-Núñez JE, Cáceres-Alvarado N, de Sanildefonso-Pereira A, Toscano-Novelle MA, García-Martínez MT, Jove-Albores P. Abdominoperineal resection in anal cancer: Reconstruction of the perineum with a myocutaneous flap from the anterior rectus abdominis muscle. Cir Esp. 2011;89(1):31-6. [ Links ]
17. Agarwal P, Sharma D. Repair of duodenal fistula with rectus abdominis muscle "pull-In" flap. Indian Journal of Surgery. 2005;67(5):253-6. [ Links ]
18. Chander J, Lal P, Ramteke VK. Rectus abdominis muscle flap for high-output duodenal fistula: novel technique. World J Surg. 2004;28(2):179-82. [ Links ]
19. Jex RK, van Heerden JA, Wolff BG, Ready RL, Ilstrup DM. Gastrointestinalanastomoses. Factors affecting early complications. Ann Surg. 1987;206(2):138-41. [ Links ]
20. Maher JW, Bakhos W, Nahmias N, Wolfe LG, Meador JG, Baugh N, Kellum JM. Drain Amylase Levels are an adjunct in detection of gastrojejunostomy leaks after Roux-en-Y gastric bypass. J Am Coll Surg. 2009;208(5):881-4. [ Links ]
21. Pickleman J, Watson W, Cunningham J, Fisher S, Gamelli R. The failed gastrointestinal anastomosis: An inevitable catastrophe? J Am Coll Surg. 1999;188(5):473-82. [ Links ]
22. Turrentine FE, Denlinger CE, Simpson VB, Garwood RA, Guerlain S, Agrawal A, et al. Morbidity, Mortality, Cost, and Survival Estimates of Gastrointestinal Anastomotic Leaks. Am CollSurg. 2015;220(2):195-206. [ Links ]
23. Nyhus LM, Wastell C. Surgery of the stomach and duodenum. 4th. ed.Boston, Toronto: Little, Brown and Company; 1986. [ Links ]
24. Siewert JR, Hölscher AH. Billroth I gastrectomy. In: Siewert JR, ed. Breitner chiururgishe operationsdre bd IV chirugie des abdomens II. Ösophagus, magen duodenum. Baltimore: Urban and Schwarzenberg; 1989. [ Links ]
25. Fischer JE, Bland KI, Callery MP. Mastery of Surgery. Lippincott Williams & Wilkins, 2006. [ Links ]
26. Hyman N, Manchester T, Osler T, Burns B, Cataldo P. Anastomotic leaks after intestinal anastomosis. It’s later than you think. Ann Surg. 2007;245(2):254-8.
27. Witz JP, Maillard JN, Tremolieres J. Fistules après gastrectomie. J Chir 1967;94:217-42. [ Links ]
28. Francillon J, Baudet B, Tissot E, Pasquier P, Vignal J. Complications post-opératoires précoces propes aux gastrectomies partielles. Chirurgie. 1975;101(10):722-33. [ Links ]
29. Tamames S, Tamames Jr. S, Martínez Ramos C, Vega DS, Nuñez Peña JR. Complications in stomach surgery. Cir Esp. 2001;69(3):235-42. [ Links ]
30. Oh SJ, Choi WB, Song J, Hyung WJ, Choi SH, Noh SH. Complications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single institute. J Gastric Cancer. 2009;13(20):239-45. [ Links ]
31. Chander J, Lal P, Ramteke VK. Rectus abdominis muscle flap for high output duodenal fistula: Novel techniques. World J Surg. 2004;28(2):179-82. [ Links ]
32. Allison JG, Cabot EB, Charles DM. Musculoepithelial flaps in the management of acute duodenal defects. Am Surg. 1983;49(6):333-7. [ Links ]
33. Yoshida E, Ohmura S, Sugiki M, Anai K, Maruyama M. A novel function of extra erythrocytic hemoglobin: identification of globin as a stimulant of plasminogen activator biosynthesis in human fibroblasts. Thromb Haemost. 2001;86(6):1521-7. [ Links ]
34. Rose D, Yarborough MF, Canizaro PC, Lowry SF. One hundred and fourteen fistulas of gastrointestinal tract treated with total parenteral nutrition. Surg Gynecol Obstet. 1986;163(4):345-50. [ Links ]
35. Lui RC, Friedman R, Fleischer A. Management of postirradiation recurrent enterocutaneous fistula by muscle flaps. Am Surg. 1989;55(7):403-7. [ Links ]
36. Chang N, Mathes S. Comparison of the effect of bacterial inoculation in musculocutaneous and random pattern flaps. Plast Reconstr Surg. 1982;70(1):1-10. [ Links ]
37. Lord MG, Valies P, Broughton AC. A morphologic study of the submucosa of the large intestine. Surg Gynecol Obstet. 1977;145(1):55-60. [ Links ]
38. Daduokis JD, Papavramidia ST, Karkavelas GN, Aidonopoulos AP, Aletras HA. Pedicle graft of peritoneum and transversalis muscle for the repair of large defects in the duodenal wall. Eur J Surg. 1993;159(1):31-3. [ Links ]
39. Yin WY, Huang SM, Chang TW, Lin PW, Hsu YH, Chao K, et al. Transverse abdominis musculo-peritoneal (TRAMP) flap for the repair of large duodenal defects. J Trauma. 1996;40(6):973-6. [ Links ]
40. Lee SJ, Lim J, Tan WTL, Baliarsing A, Lau PTC, Tan LKS, et al. Changes in the local morphology of the rectus abdominis muscle following the DIEP flap: an ultrasonographic study. Br J Plast Surg. 2004;57(5):398-405. [ Links ]
41. Erni D, Harder YD. The dissection of the rectus abdominis myocutaneous flap with complete preservation of the anterior rectus sheath. Br J Plast Surg. 2003;56(4):395-400. [ Links ]
Correspondence:
Juan A. Díaz-Plasencia, MD, Av América Sur 3145, Trujillo 1075, Trujillo, Perú.
E-mail: alberdiaz@hotmail.com
Recibido: 15.05.18
Aprobado: 15.04.19