INTRODUCTION
A relationship between Irritable Bowel Syndrome (IBS) and Celiac Disease (CD) has been demonstrated by several authors (1,2). IBS is the most common gastrointestinal disorder in the West. In Uruguay, Iade et al. (3) documented a prevalence of 14.8% in women and 5.4% in men in a general population sample in the capital city of Montevideo. There is no data on the prevalence of IBS among celiac patients in Uruguay. The occurrence of IBS symptoms in CD has been touted as yet another piece of evidence in support of an inflammatory pathogenesis in IBS.
While the pathophysiology of IBS has not been elucidated, available evidence suggests roles for gut motor dysfunction, visceral hypersensitivity and inflammatory mediators, among others 4. Indeed, a considerable body of literature indicates that enteric infections, as well as intestinal inflammation, can alter the function of the gastrointestinal tract 5. Thus postinfection IBS has been well documented and post-viral syndromes, such as gastroparesis, provide a further example of infection-associated or initiated dysmotility. Direct evidence relating to the role of inflammation in IBS and related disorders is obtained first and foremost from inflammatory bowel disease, where motility is known to be altered 6. In these patients, some of the motor abnormalities persist after apparent resolution of the inflammatory process, leading some to suggest an evolution towards a syndrome analogous to IBS during periods of inflammatory remission 6. At a more fundamental level, chronic inflammation, such as that seen in inflammatory bowel diseases, has been shown to produce significant changes in the function and phenotype of gut smooth muscle and enteric neurons, generating a self-perpetuating cycle of interaction between inflammatory cells and the neuro-muscular apparatus of the digestive tract 6.
Several lines of evidence indicate that inflammation and cytokine imbalance may contribute to the pathogenesis of symptoms of IBS 7. In fact, proinflammatory cytokines such as IL-1α, IL-6, IL-8, TNFα are increased in patients with IBS 8-10. Among these cytokines, IL-6 and IL-8 have been among those most consistently found to be increased in sera of patients with IBS 11. Since the levels in sera of these cytokines seem to correlate with IBS symptoms, they might be considered as good markers of the disorder. Published data on IL-12 levels in IBS patients are conflicting: two studies 12,13 did not find any differences in serum IL-12 levels between IBS patients and controls, while another study 14 found increased levels of IL-12 in pediatric IBS patients compared to healthy controls.
In celiac disease, inflammatory mediators generated by gluten ingestion have been invoked in the induction of IBS symptoms 1,15,16 .
The aim of this study was to determine if there is an association between the inflammatory process in celiac patients and IBS symptoms.
Considering that systemic inflammation and elevated levels of circulating pro-inflammatory cytokines have been linked to depression and impaired quality of life in IBS patients, we also explored, in these CD patients, correlations between quality of life, IBS symptoms and markers of inflammation 17,18.
MATERIALS AND METHODS
A cross-sectional study was performed in patients with CD.
Study population
Patients previously diagnosed with CD based on the presence of at least one positive antibody (tissue transglutaminase (IgA tTG) or endomysial (IgA EMA)) and histological findings (Marsh criteria Stage II, III or IV) were included. Those under 18 years of age, who voluntarily decided not to participate or were pregnant were excluded.
Socio-demographic data and medical history were collected in all patients.
Serological markers value
Two groups were defined: "positive" (at least one positive antibody - IgA tTG or IgA EMA or IgA + IgG GDP at recruitment) and "negative" (all negative serological markers at recruitment). The determination of IgA tTG IgA EMA and IgA + IgG GDP antibodies was performed using commercial kits (Inova Diagnostic Inc, San Diego, California, USA).
IBS diagnosis
The presence of IBS was assessed by a Spanish version of the Rome II Modular Questionnaire (RIIMQ). IBS was considered when there was abdominal pain or discomfort for at least 12 weeks in the last 12 months that improved with bowel movement and/or was associated with a change in defecation frequency and/ or a change in stool consistency. Patients with IBS were then classified as diarrhea-predominant IBS (IBS-D) or constipation-predominant IBS (IBS-C), whereas the individuals that did not fit criteria for either IBS-D or IBS-C were classified as alternating IBS (IBS-A).
Adherence to GFD
It was assessed by self-report over a period of at least six months. Non-adherence was considered when patient reported a lack of strict adherence to GFD over a 6-month period before inclusion. Patients who reported strict adherence for less than six months were also considered non-adherent.
Evaluation of cytokine levels in serum
IL-6, IL-8 and IL12/23p40 levels in sera from all CD patients and controls were determined by specific sandwich ELISAs (BD Bioscience).
Health-Related Quality of Life
HRQoL was assessed by SF-36 questionnaire, previously validated in the Uruguayan population 19,20.
Controls
Controls were volunteers among hospital workers who were matched wth CD patients for sex and age distribution and who did not have CD (assessed by a negative IgA tTG antibody test in the presence of normal levels of IgA) and who did not meet criteria for IBS according to the Rome II Modular Questionnaire.
Ethical aspects
The approval of the Ethics Committee of the School of Medicine was obtained. Both patients and controls entering this study were asked to provide written informed consent and were informed that they were free to leave the study at any time or to refuse to enter it without any impact on their medical care. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki.
Statistical Analysis
From the data generated descriptive statistics were generated for all measures. The prevalence of IBS was calculated in the population studied. The distribution of independent variables was evaluated both in “positive” and in “negative” groups. Statistical tests were performed to compare means and proportions (t test and Chi square for continuous and discrete variables, respectively) and Odds ratios and 95% confidences intervals were calculated. The sample size calculated was of 116 subjects based on an expected prevalence of IBS in celiac patients of 26% (±8%) at a confidence level of 95%. Calculations were performed using the statistical package OpenEpi version 3.01 Cytokine data were analysed with GraphPad Prism 5. Differences in cytokine levels were considered statistically significant at a p<0.001 when analysed by one-way Anova.
RESULTS
Demographics
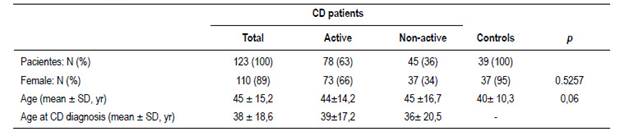
One hundred twenty-three celiac patients were included. Table 1 shows their socio-demographic and medical data. The median time on a GFD was 3.7 years (first quartile 2 years and third quartile 6 years). Seventy-eight patients were deemed to be “positive”. Among these, in 15 patients (19%) DGP was the only antibody positive, in 11 (14%) tTG was the only one positive, in none was EmA alone positive and in 54 patients (69%) more than one antibody was positive. Thirty-nine controls were included, 37 were female and their mean age was 40 yrs ± 10.3 (Table 1). IBS prevalence and IBS subtypes in each one
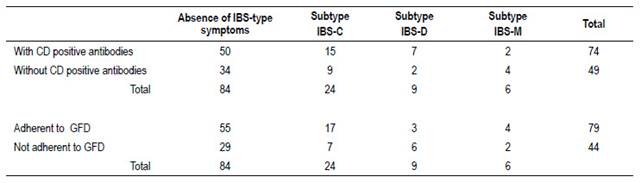
Thirty-nine patients (32%) had symptoms meeting criteria for IBS; 62% were subtype IBS-C. Table 2 shows patient distribution according to subtype and relationship to CD activity.
The occurrence rate of IBS symptoms was similar among “positive” and “negative” CD patients (OR 1.207; 95% CI 0.5636 to 2.584) (Table 2).
Adherence to GFD
In adherent patients the median time on a GFD was 3.7 years (first quartile 2 years and third quartile 6 years). Of the 79 patients that were adherent, 39 (49%) had positive antibodies. Of the 44 patients that were non-adherent, 34 (77%) had positive antibodies
The occurrence of IBS-type symptoms was not different between patients who were not adherent or were strictly adherent to GFD (OR 0.8436; 95% CI 0.3843 to 1.852)
Cytokine levels
When analyzing the systemic immune response, we observed that all CD patients had increased levels of the inflammatory cytokines IL-6, IL-8 and IL12/23p40 in sera as compared to controls (Figure 1). Furthermore, we found higher levels of these inflammatory cytokines in the sera from CD patients with IBS symptoms in comparison to those CD patients without IBS symptoms (Figure 1). However, we did not observe any difference in the levels of these cytokines when both groups were classified according to antibody positivity (Figure 2). These results suggest that there is a higher level of inflammation or immune activation in CD patients with IBS symptoms, independent of CD antibody status.
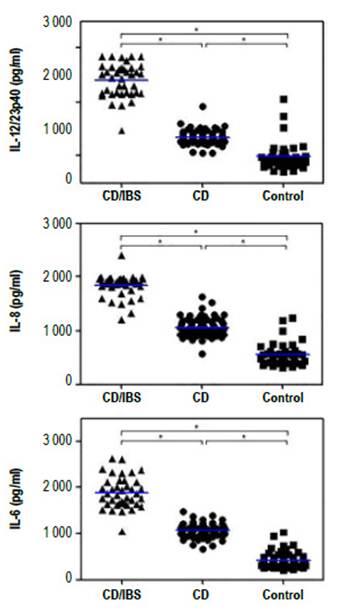
Figure 1 Cytokine levels in sera from CD patients with (CD/IBS) and without (CD) IBS symptoms and non-celiac controls (control). The levels of IL-6, IL-8 and IL12/23p40 in sera was evaluated with specific ELISAs. The asterisks represent statistically differences with p<0.001 analyzed with 1 way Anova
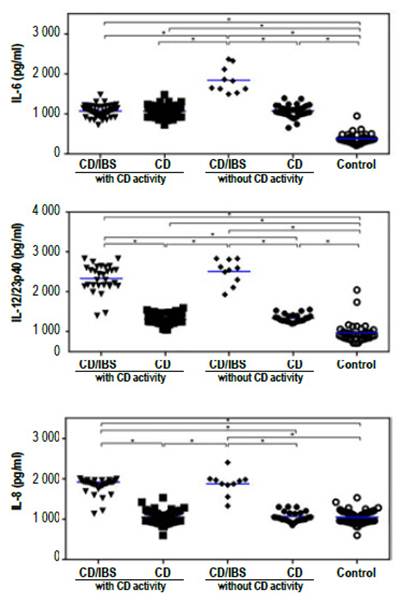
Figure 2 Cytokine levels in sera from CD patients with (CD/IBS) and without (CD) IBS symptoms classified according to the presence or absence of CD activity. The levels of IL-6, IL-8 and IL12/23p40 in sera was evaluated with specific ELISAs. The asterisks represent statistically differences with p<0.001 analyzed with 1 way Anova.
Quality of life (QoL)
A significant negative correlation between the physical component of the quality of life instrument and IL12/23p40, but not IL-6 or IL-8 levels, was found (Figure 3A). Moreover, this negative correlation was associated with the presence of CD activity (Figure 3B).
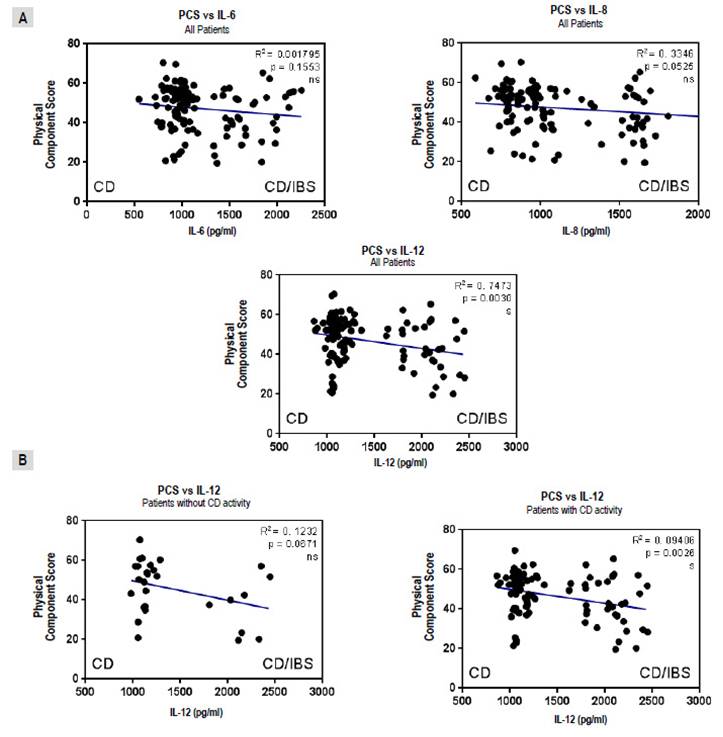
Figure 3 Correlations between physical component score (PCS) of quality of life instrument and interleukins (IL) for all patients with celiac Disease (CD) (A) and comparing IL12/23p40 levels for those in “positive” and “negative” groups (B). Results were plotted with Graphpad Prism 5.0 with the linear regression tool.
On the other hand, a significant negative correlation between the mental component and IL-6 and IL12/23p40 levels was found, but not with IL-8, (Figure 4A) that was, in turn, associated with CD activity (Figure 4B).
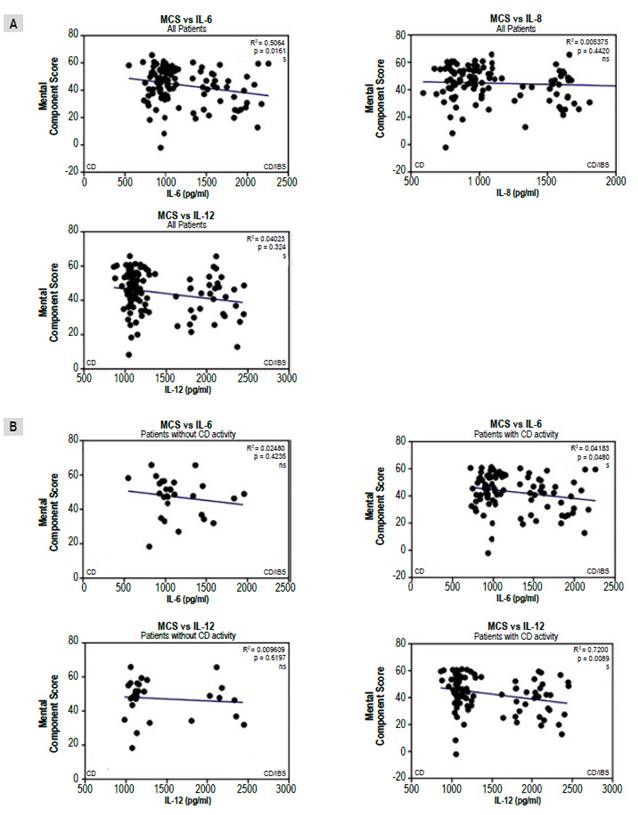
Figure 4 Correlations between mental component score (MCS) of quality of life instrument and interleukins (IL) for all patients with celiac Disease (CD) (A) and comparing IL-6 and IL12/23p40 levels for those in "positive" and "negative" groups (B). Results were plotted with Graphpad Prism 5.0 with the linear regression tool.
DISCUSSION
IBS occurred in 32% of this group of Uruguayan celiac patients, with a statistically significant predominance of the constipated subtype. The occurrence rate for IBS was not different between those defined as "positive" and "negative" for CD antibodies. CD patients had increased levels of serum IL-6, IL-8 and IL12/23p40 compared to controls. These levels were higher in patients with IBS than those without IBS. No difference was found between "positive" and "negative" patients with respect to cytokine levels. However, cytokine levels were predictive of impaired quality of life and, especially so, among those with evidence of disease activity.
The prevalence of IBS in this group of celiac patients was higher than that previously reported in the general population in Uruguay 3. A meta-analysis reported a prevalence of IBS in CD patients of 20 to 58.4% 21. The authors attributed this wide range to considerable heterogeneity between studies. When they considered only studies that used the Rome criteria, the prevalence was 41% 21; higher than in this group of patients.
The distribution of IBS subtypes was similar to that described in the general population in Uruguay, with a predominance of IBS-C (3).
It is possible that mucosal inflammation induced by gluten may trigger IBS symptoms in patients with known CD. Monitoring mucosal inflammation (disease activity) in treated CD patients remains a challenge. Although specific antibodies are highly sensitive and specific in the diagnosis of CD, some studies had suggested that they do not correlate well with histological findings in celiac patients on a GFD (22). Nevertheless, a recent study23, demonstrated that elevated levels of DGP IgG, DGP IgA and TTG IgA antibodies were highly predictive of persistent villous atrophy. DGP IgA had previously been found to be accurate in predicting persisting villous atrophy, and Mohadev et al. confirmed that, when positive, DGP serology predicts the persistence of abnormal histological findings 23,24. Nonetheless, we did not find a relationship between the occurrence of IBS symptoms and antibody status in these celiac patients.
The truly novel aspect of this study was the measurement of inflammatory cytokines in the various patient groups. We found, firstly, that CD patients, in general, had higher levels of pro-inflammatory cytokines (IL6, IL8 and IL12/23p40) than controls and, secondly, and most interestingly, that celiac patients with IBS showed higher levels of each cytokine than celiac patients without such symptomatology. However, we failed to detect any relationship between cytokine levels and celiac disease antibodies. Cytokine levels were also predictive of impaired quality of life.
Most studies pertaining to cytokine elevations in CD have been performed using duodenal or jejunal biopsy samples with measurement of cytokine production at a local level in small bowel mucosa 25-27. At the systemic level, a limited number of studies have assessed serum cytokines. Thus, increased levels of IL-2, IL-4, IL-10, IL-18, IFNy and TNFα have been demonstrated in the antibody "positive" patients 28-30. Manavalan et al. 26 found, in a small group of celiac patients, that those with active CD, as well as those on a GFD but with positive antibodies, had significantly higher levels of various cytokines: IFNg, IL-1β, IL-6, IL-8, IL-4 and IL-10 in comparison to controls and patients with negative antibodies. They also found that there was a statistically significant correlation between levels of TTG IgA antibodies and serum levels of IL-4, IL-10, IL-1α, IL-1β and IL-8.28
In addition to IL-6 and IL-8, we found elevated serum levels of IL-12/23p40 among CD subjects. Vazquez Frias et al. 14 also found an increased level of IL-12 in IBS compared to healthy controls but in a pediatric population. However, Leon and colleagues, found that IL-12/23p40 levels in the small bowel mucosa among CD patients were normal regardless of disease activity 24. The the IL-12 family of cytokines, including IL-12 and IL-23, are important mediators of immune-mediated inflammatory diseases such as psoriasis, multiple sclerosis, rheumatoid arthritis, and Crohn’s disease 31,32, through the induction of T helper 1 differentiation.
Several studies have reported the presence of elevated levels of IL-6 and IL-8 and other proinflammatory cytokines, such as TNFα, in certain IBS populations 9-12,33-35. While several systematic reviews have linked IBS to various alterations in serum levels of certain cytokines (both increased and decreased) 33,34, results have been variable. In these various studies, elevated levels of IL-6 and IL-8 and reduced levels of IL-10 have been relatively frequent findings 9-11,14,25. In one study, elevations of TNFα were found but confined to those with systemic comorbidities 11. Given the heterogeneity of any IBS population, such variability is to be expected and it may well emerge that these inflammatory markers are a feature of a specific IBS sub-population whose precise nature remains to be defined. That these cytokine perturbations might be relevant to IBS pathophysiology is suggested by two studies linking pro-inflammatory molecules 35 and IL-6, in particular 36, with neuronal stimulation and visceral hypersensitivity in animal models of IBS.
Our findings in this study provide further support for a role for certain cytokines in IBS. Thus, while we found that elevated levels of IL-6 and IL-8 were a feature of those CD subjects who exhibited symptoms compatible with IBS, these elevations were unrelated to the inflammatory process in CD. This finding is consistent with a recent report that associated a similar cytokine profile with the presence of IBS symptoms among a group of patients with ulcerative colitis in deep remission 37.
Furthermore, we have found that there was a significant correlation between inflammatory cytokines and quality of life of IBS patients. Indeed, circulating levels of IL12/23p40 in "positive" CD patients correlated with a decrease in the physical component of quality of life, while high plasma levels of IL-6 and IL12/23p40 in "positive" CD patients were associated with a decrease in the mental component of quality of life. Furthermore, CD patients with IBS symptoms presented higher levels of IL-6 and IL12/23p40, indicating that IBS symptoms are associated with an increase in the inflammatory response and a decrease in quality of life of CD patients. Our results are in line with those of O’Leary et al who found that the presence of IBS symptoms was the strongest predictor of impaired QOL in celiac disease 1. In another study, Chang et al found that the serum levels of IL-1β, IL-6, IL-8, IL-10, IL-12, and TNFα had no significant correlation with quality of life in IBS patients 12. However, more recently, Choghakhori et al. 38 found a significant correlation between inflammatory cytokines and quality of life of IBS patients and lower IL-10 serum levels were significantly correlated with lower IBS-QoL.
Gut microbiota-related systemic inflammation (mediated by pro-inflammatory cytokines, among others), may trigger neuroinflammation and lead to dysfunction in such brain regions as the hippocampus and cerebellum; together with a dysfunctional vago-vagal gut-brain axis, these changes may promote cognitive impairment 10.
In summary, in this CD population, IBS symptoms were common, but their occurrence was unrelated to antibody status. Higher levels of inflammatory cytokines and lower quality of life were found in patients with IBS symptoms than in both those without such symptoms and control subjects, but the detection of elevated serum cytokine levels was not related to CD antibody status, suggesting that IBS symptoms in CD, though related to cytokine changes, relate to a different inflammatory process than that which typifies celiac disease.