INTRODUCTION
Pneumatosis intestinalis (PI) is the presence of gas within the intestinal wall. It represents an imaging or surgical finding, and not a pathological entity per se 1.
It is classified as primary (15%) when no possible etiological factor is identified, and as secondary (85%) in association with a wide variety of conditions and pathologies, including asthma, trauma, intestinal ischemia, abdominal infections, medications, immunosuppression, and inflammatory/autoimmune diseases, among others 2.
Its pathogenesis is unclear. However, theories have been proposed to explain its formation. The proposed mechanisms include damage in the intestinal mucosa, gas generation by intestinal bacteria, and gas from the thorax in relation to pulmonary diseases 3.
It may be asymptomatic or have nonspecific gastrointestinal symptoms. Management depends on the benign or catastrophic nature of its clinical presentation 1-3.
CASE REPORT
Eighteen-year-old female patient with a history of intermittent episodes of chronic abdominal pain located in the right half of the abdomen, predominantly in the ipsilateral iliac fossa, and nausea since the age of 14. During the last 2 years, she presented increased frequency of abdominal pain, associated with abdominal distension, emesis and episodes of diarrhea without blood, lasting 2 days, which occurred 2-3 times a month, without identified triggers.
In the last 2 years she had undergone endoscopic studies of the upper and lower gastrointestinal tract, with no pathological findings; 18 months ago, she had an abdomen CT scan that showed thickening of the jejunal wall and dilation of the small intestine loops. She had not received any diagnostic intervention in this regard.
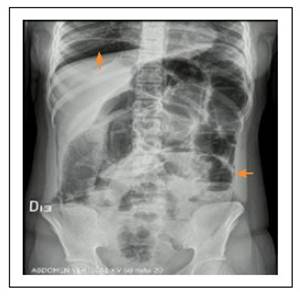
She was admitted to the emergency department of our institution for worsening of abdominal pain, increased abdominal distension, emesis of food content and absence of stool for 5 days. Her physical examination showed normal vital signs, mild dehydration, abdominal distension, and positive Blumberg sign. Her low height and weight (BMI 16) attracted attention. Paraclinical tests showed mild leukocytosis; simple abdominal x-ray showed evidence of dilation of the small intestine loops, with stack of coins appearance, hydroaerial levels and pneumoperitoneum (Figure 1). Due to suspected intestinal obstruction with possible hollow visceral perforation, the patient underwent diagnostic laparoscopy. On entering the abdominal cavity, the exit of abundant gas and citrine peritoneal fluid was observed. Conversion to infraumbilical laparotomy was necessary due to technical difficulties, given the presence of dilated intestinal loops. Dilation of intestinal loops, PI of the jejunum, wall erythema and mesenteric adenitis were documented, and no perforations were found.
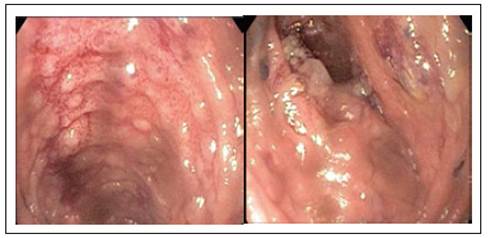
Contrast abdominal CT was performed showing segmental thickening of the jejunum and ileum walls, mesenteric adenitis, and PI of the jejunum. Due to suspected inflammatory bowel disease (IBD), ileocolonoscopy was performed without abnormal findings. It was decided to complement the diagnostic study with a magnetic resonance enterography (Entero-MRI) of the abdomen that revealed findings similar to those described in the CT (Figure 2); due to this, enteroscopy was performed that showed evidence of ulcerated ileitis (Figure 3). The histological study revealed flattening of the villi, abundant lymphocyte-dominated infiltration of the lamina propria with polymorphonuclear cells permeating the glandular epithelium. PAS, ZN stains were negative. PCR for cytomegalovirus (CMV), herpes, cultures for common germs and for tuberculosis mycobacteria were also negative. Fecal calpropectin level was elevated (1,196 mcg/g).
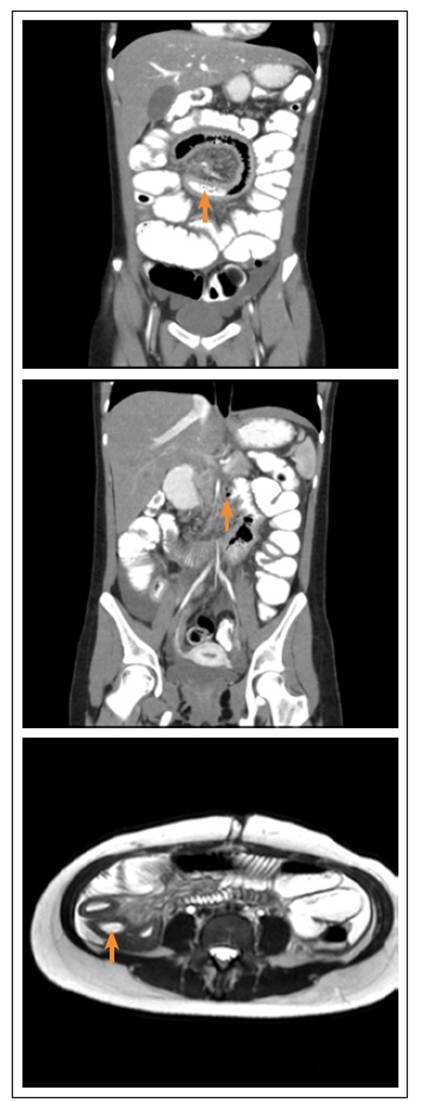
Figure 2 Above and center: abdominal CT scan, thickening of the jejunal wall, pneumatosis within the jejunal wall. Below: Entero-MRI, thickening of the ileum wall.
With all the clinical, imaging, endoscopic and histological findings, the diagnosis of Crohn s disease (CD) IBD type was confirmed, and systemic steroid and immunomodulatory management was instituted. Four weeks after starting therapy, the patient reported 80% improvement in her symptoms, and with the intervention of clinical nutrition, she managed to gain 3 kg of weight. The patient is in plan of starting biological medication.
DISCUSSION
PI is defined as the presence of gas within the intestinal wall. It can be cystic, microvesicular or linear in appearance, and is located in the submucosal or subserosal planes 1. Cysts are filled with hydrogen, nitrogen, and carbon dioxide. It was first described in 1783 by Du Vernoi as a finding of pathological anatomy 4. It occurs in any age group and its incidence is approximately 0.03% 3, although case reports tend to increase, related to the wide use of CT in the emergency department and the improvement in sensitivity of the study. Although it can occur anywhere in the gastrointestinal tract, PI is most frequently located in the colon (46%), followed by the small intestine (27%), both in the colon and in the small intestine (7%), and in the stomach (5%) 3,5.
The mechanism by which PI occurs is unclear. Proposed hypotheses include: 1) mechanical theory: intraluminal gas enters the intestinal wall due to mechanical forces through ruptures of the mucosa, 2) Bacterial theory: gas-forming bacteria (such as Clostridium) penetrate the intestinal mucous membrane, producing gas that is retained in the submucosa and lymphatic vessels, 3) pulmonary theory: chronic pulmonary diseases produce alveolar rupture and as a consequence, emphysema in mediastinum with air migration along the aorta and mesenteric vessels to the abdominal wall 2,4. In our patient, the chronic inflammatory process of the mucosa associated with Crohn s disease contributed to the pathophysiological mechanism.
More than a pathological entity, PI is a radiological finding that has been described in association with a broad spectrum of pathologies or conditions (secondary) in 85% of cases; in the other 15% the etiology is not identified (primary or idiopathic) and implies a benign chronic condition 1,3,4.
Based on the evaluation of multiple case reports, 6 Feuerstein documented more than 50 different etiologies, grouped into gastrointestinal, infectious, medicinal (iatrogenic), neoplastic, pulmonary, rheumatic and transplant-associated (Table 1).
Table 1 Causes of pneumatosis intestinalis. of non-surgical pneumoperitoneum (11). Our patient
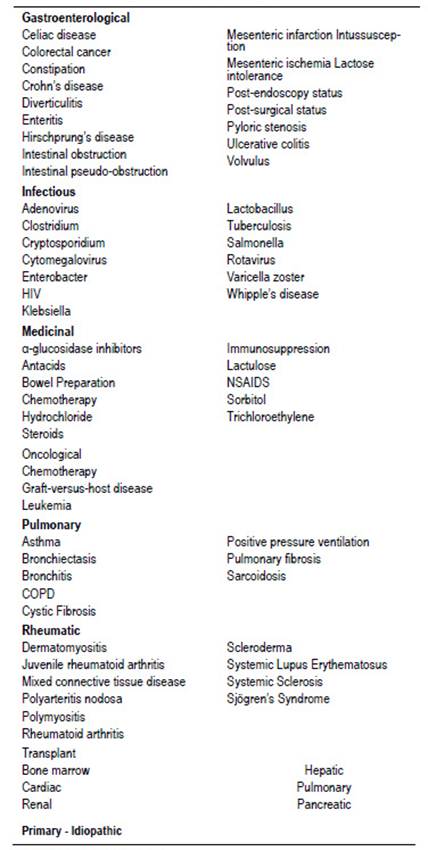
Taken from Feuerstein 2014.
Our patient was diagnosed with Crohn s disease after several years with a clinical picture suggestive of the disease. The association of PI with Crohn s disease does not require interventions in addition to those performed for the underlying pathology, unless complications such as those described below occur.
Most cases of PI are asymptomatic, so they are diagnosed as an incidental radiological or endoscopic finding. Patients may present with nonspecific gastrointestinal symptoms: mild abdominal pain (59%), diarrhea (53%), weight loss (55%) nausea and vomiting (14%), mucus or blood in the stool (12%) 1,3,4,7.
Generally, patients with primary PI have hemodynamic stability, normal lactate levels, and no signs of sepsis. In contrast, patients with life-threatening conditions often present with signs of peritoneal irritation 1,2. In general terms, PI symptoms are varied, and depend on the location of the pneumatosis and the underlying etiology.
CT is the most sensitive test for diagnosing PI. Some authors have described an association between the linear pattern of PI and intestinal ischemia compared to the benign nature of the cystic pattern. However, none of these patterns is pathognomonic of any underlying pathology, and they have not been correlated with its severity or etiology 8. CT also allows to detect findings that may suggest the underlying etiology, as well as potentially worrisome findings (red flags), such as thickening of the intestinal wall, decreased uptake of contrast material by the mucosa, dilation of intestinal loops, ascites, or presence of portal gas 2,9. Our patients abdominal CT showed thickening of the jejunum and ileum walls and dilation of the loops of the proximal small intestine, so she underwent additional diagnostic studies.
Between 3 and 16% of patients with PI present complications, which are classified as intestinal or extraintestinal 2. Intestinal complications are caused by the growth or rupture of cysts into the peritoneal space (intestinal obstruction, pneumoperitoneum). Extraintestinal complications are secondary to adhesions or to compression of adjacent organs by large cysts 10. In pneumoperitoneum it is a radiological finding that has multiple underlying etiologies; in more than 95% of cases it is secondary to the rupture of a hollow viscera, so a surgical intervention is required. PI is the main cause presented with an acute abdomen; the abdominal x-ray was compatible with pneumoperitoneum and signs of intestinal obstruction, so we decided to operate on her. The patient’s pneumoperitoneum is explained by the rupture of the cysts into the peritoneum.
A colonoscopy is often necessary to exclude intestinal lesions and to perform biopsies. The endoscopic appearance of PI consists of cystic lesions with surrounding atrophic or reddened mucosa 2. In our patient, an enteroscopy was performed to evaluate the jejunum and ileum mucosa and performing a biopsy, taking into account that the findings of the Entero-MRI and the clinical presentation suggested Crohn s disease in this location.
There is no unified treatment for PI, and surgical intervention is not absolutely necessary. Management depends on the etiology and severity of symptoms. In the case of an incidental finding and benign presentation, conservative management is preferred. Cases managed with clinical surveillance, antibiotic therapy and hyperbaric oxygen therapy have been described (the increase in blood partial pressure of oxygen increases the gas pressure gradient with respect to the cysts, which release the gases contained within them and are recharged with oxygen, which is subsequently metabolized and leads to resolution). For cases presenting as an acute intra-abdominal catastrophe, surgical treatment should be considered 1-3,9.
Several authors have attempted to identify ominous indicators that require surgical intervention. In a prospective review of patients with PI by Knechtle, the following findings predicted intestinal necrosis requiring surgery: acute abdomen, metabolic acidosis, elevated serum lactate or amylase, and presence of portal venous gas 12. On the other hand, Greenstein documented age over 60, presence of emesis and leukocytosis over 12 000/mm³ as independent risk factors for surgical management. In this study, the main independent predictor of mortality was the presence of sepsis 13. In 2013, DuBose conducted a multicenter retrospective study with 500 patients in which he identified lactate greater than 2 mmol/L, low blood pressure or vasopressor requirement, peritonitis, acute kidney injury, mechanical ventilation, absence of bowel sounds, and the presence of portal vein gas as independent predictors of risk for pathological pneumatosis (defined as that requiring surgical intervention) 14.
In our patients case, acute abdominal pain with signs of peritoneal irritation, imaging compatible with intestinal obstruction and pneumoperitoneum, and leukocytosis, suggested an intra-abdominal catastrophe with indication for surgical management. In conclusion, PI may occur in patients with Crohns disease; this is a rare radiologic finding that should be interpreted and studied in the context of its clinical presentation. Imaging studies have a role in identifying the underlying etiology and the red flags indicating surgical intervention. The management is directed to that of the underlying pathology. If no serious complications occur, the prognosis is good.