INTRODUCTION
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and ulcerative colitis (UC). They are both chronic inflammatory disorders of the gastrointestinal tract, of yet unknown etiology, and are characterized by chronic inflammation of the intestinal wall. The diagnosis of IBD is generally established during a flare based on the clinical symptomatology, endoscopic aspect, pathological study and the evolutionary profile under treatment 1. Medical therapy is mainly based on therapies aiming to reduce the inflammatory response, such as corticosteroids, immunomodulators, and biological drugs 2,3. Treatment regimens for IBD incorporate the use of a variety of immunosuppressive agents (thiopurines, methotrexate, cyclosporine, anti-TNF-alpha agents).
The development of these treatments has reduced the need for surgery, the number of hospitalizations and improved the chances of deep remission 4. However, immunosuppressive drugs increase the risk of infections, hepatitis B and C reactivation especially when used in association.
It is also recognized that patients followed for IBD would be at risk for hepatitis B and C infections due to the extensive use of endoscopic procedures, blood transfusions, and surgery. Tunisia is considered a low- endemicity area for both hepatitis B and C viral infections. The prevalence of HBs Ag in Tunisia is indeed 1.8% while it is estimated at 3.5% worldwide 5,6. The prevalence of anti HCV Ab is 0.87% versus 1% worldwide 7,8.
The aim of our study was to assess the prevalence and the characteristics of hepatitis B and C viruses in Tunisian IBD patients on immunosuppressive treatments, and to determine the associated factors to these viral infections.
MATERIALS AND METHODS
Study design
A retrospective study including all consecutive patients with IBD (CD or UC) under immunosuppressive therapies (thiopurines, methotrexate, cyclosporine, anti-TNF-alpha agents) was performed at a tertiary-level hospital (Habib Thameur Hospital, Tunis) from January 2017 to December 2018. This study was performed according to the declaration of Helsinki, following the guidelines for good clinical practice and the ethics committee approved the study protocol. Data were collected, and handled anonymously. The diagnosis of IBD was based on clinical, endoscopic, histological, radiologic, and therapeutic criteria. We included all patients with IBD (CD or UC) who were screened for viral hepatitis C (anti HCV Ab) and viral hepatitis B (at least the search for the HBs antigen (HBs Ag) and anti HBc antibodies (anti HBc)). A total of 74 IBD (62 CD and 12 UC) patients were included. The following variables were recorded: age, gender, disease duration, immunosuppressive therapies, previous surgery related or not to IBD and disease distribution according to the Montreal Classification.
HBV and HCV screening
Patients were tested for HBs Ag, antibodies to hepatitis B core protein (anti-HBc), and antibodies HCV (anti-HCV Ab), using an enzyme-linked immunosorbent assay. HBs Ag-positive sera were tested for hepatitis B e antigen (Hbe Ag), and antibodies to HBe Ag (anti-HBe). If HBs Ag and/or anti-HBc were positive, HBV viral load was analyzed by the PCR Real-Time. Anti-HCV-positive antibodies sera were tested for HCV RNA using the same technique.
Statistical analysis
The data were analyzed on the Statistical Package for the Social Sciences (SPSS) version 20, IBM SPSS Inc.; Chicago, IL, USA). Results were expressed as mean ± standard deviation. Univariate comparisons between groups were performed using chi-squared tests for dichotomous variables or Fisher’s test when appropriate. For continuous variables, independent samples t-tests were used. Potential associated factors to HBV/HCV infection such as gender, age, type of disease, therapy, disease duration for a positive or negative testing result, and univariate logistic regression models were used. Multivariate models have been tested for the same outcome. A p value of <0.05 was considered to be significant.
RESULTS
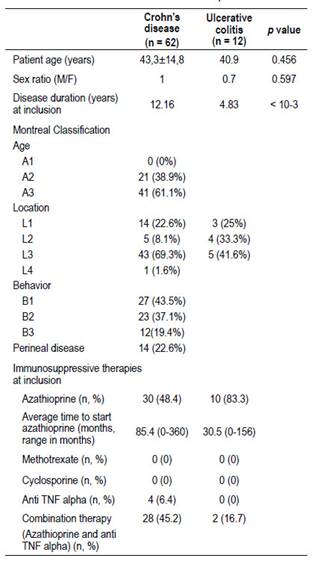
Mean age of the 74 IBD patients at study inclusion was 43.53 ± 14.2 years (range, 17-73). Of these patients, 38 (51.4%) were female, and 36 (48.6%) were male. The median disease duration at study inclusion was 9 years (IQR, 6-13). Of the 74 patients, 83.8% were diagnosed with Crohn’s disease and 16.2% with ulcerative colitis. Among the 62 CD patients, 30 were on azathioprine alone (48.4%), four on infliximab alone (6.4%) and the other 28 (45.2%) were on combination therapy (azathioprine with infliximab or adalimumab). The average duration of treatment with azathioprine was 54.5 months (range, 6 to 180 months). The average time to start treatment with azathioprine was 85.4 months (0 - 360 months). Thirteen patients were on infliximab and only two patients received adalimumab. The average time to initiate anti-TNF alpha therapy was 119.3 months (0 - 348 months). Of the 12 patients with UC, 10 were on azathioprine (83.3%), and two (16.7%) on combination therapy (azathioprine with infliximab). The average time to start azathioprine was 30.5 months (0 - 156 months).
Demographic, clinical, and therapeutic characteristics of the study cohort are reported in Table 1.
Prevalence of HBV markers
Present HBV infection (HBs Ag positive) was found in 1.3% of the general population, 1.6% of CD, and 0% of UC patients. There was no significant difference in prevalence of HBV among male and female patients. The prevalence of anti HBc in CD and UC patients were 9.7% and 0% respectively (p=0.260). In general IBD population, HBc antibodies prevalence was 8.1%.
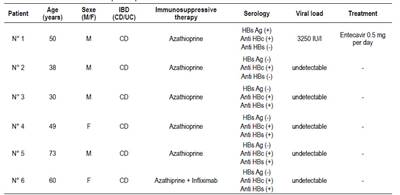
Patient 1 had active chronic hepatitis, and received entecavir. Number 2 CD Patient had only anti-HBc, and viral load was negative (occult infection). Anti-HBs antibodies were associated with anti-HBc in 4 patients (patients 3, 4, 5 and 6). Anti-HBs prevalence in UC patients (58.3%) was significantly higher than in the CD group (19.3%, p=0.004). The characteristics of the 6 HBV-positive patients were summarized in Table 2.
Anti-HBV Vaccination Status
Effective vaccination was detected in 15 (20.2%) of the 74 IBD patients. The vaccination rate in negative HBV patients, defined as anti-HBs antibody level ≥ 10 mIU/ml without the presence of HBc antibodies, was 22%.
Prevalence of HCV infection:
HCV infection (HCV Ab positivity) was found in 4% of all patients (CD: 3.22%, UC: 8.3%). There was no statistical difference between CD and UC (p=0.411;OR 0.366; 95% CI 0.03-4.40). The all 3 patients were positive for HCV RNA (Table 3). At univariate logistic regression, we didn’t found any association between HCV infection and age, gender, former steroid use, use of combination therapy and surgery. Also no significant difference was found in disease locations based on Montreal Classification. Two patients (69-years-old woman and 34-years-old man) developed non severe acute hepatitis C. The other patient had chronic hepatitis C, treated with pegylated interferon and ribavirin. Only one patient received direct-acting antivirals (DAA) (available in Tunisia since 2015), the other patient were treated before the advent of DAA in Tunisia.
Table 3 Characteristics of the 3 HCV-positive patients.
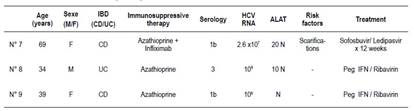
M: male, F: female, CD: Crohn’s disease, UC: ulceratice colitis, HCV: Hepatitis C virus, Peg IFN: pegylated interferon
Comparison between patients with positive and negative HBV/HCV markers (Table 4)
Table 4 Comparison between patients with positive and negative HBV/HCV markers.
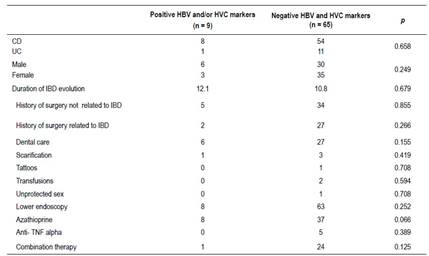
M: male, F: female, CD: Crohn’s disease, UC: ulceratice colitis, HBV: Hepatitis B virus, HCV: Hepatitis C virus
When comparing patients with positive HBV and/or HCV markers with patients with negative markers, there was no significant difference concerning patient gender, type or the duration of IBD evolution, and different hepatitis risk factors (history of surgery related or not to IBD, dental care, scarification, tattoos, transfusions, unprotected sex).
Risk of viral reactivation under immunosuppressive therapy
No clinical or biological sign of HBV reactivation was observed during azathioprine on monotherapy, biologics or combination therapy. For patient 1 and during azathioprine therapy, no clinical or biological signs of reactivation were observed under NUC (entecavir). He had been receiving entecavir since hepatitis B infection diagnosis. For HCV patients, two patients out of the 3 with positive HCV serology was HCV RNA-positive and presented with non severe acute hepatitis (ALAT > 10 N). Patient 7 was followed for CD, and received azathioprine and infliximab. Patient 8 was also followed for CD and had been treated with azathioprine. Under antiviral treatment and withdrawal of immunosuppressive therapy, outcome was favorable with normalization of liver enzymes and sustained virological response (SVR). Patient 9 had chronic HCV infection, RNA was detectable (106 IU/l) without cytolysis and liver fibrosis was observed (F2 in Fibroscan®). Sustained virological response was obtained with peg IFN and ribavirin.
DISCUSSION
In current study, we found that the prevalence of HBV infection did not differ between patients with IBD and the general population but HCV prevalence is higher in IBD patients than general population.
Despite screening and elimination programs, hepatitis B and C viral infections remain a public health problem. In 2015, the global prevalence of chronic hepatitis C was estimated to be at 1% (71 million chronic carriers of the hepatitis C virus worldwide). The regions with the highest prevalence are represented by the Middle East (2.3%), and Europe (1.5%) 7. In Tunisia we report national- level prevalence estimates of chronic HBV at 1.8%.
It is estimated that 257 million people worldwide have chronic hepatitis B infection (global prevalence of 3.5% in 2016) 5. The most affected regions are the Western Pacific, and Africa, where 6.2% and 6.1% of the adult population is infected respectively. About 65 million women of reproductive age are infected and at risk transmit infection to their children. In the Maghreb region, an estimated 2.7 million people (1.8 to 4.7%) had chronic HBV infection 5.
The prevalence of HBV infection in our cohort is 1.3% (1.6% in CD and 0% in UC) is similar to that reported in a recent Indian study, which found 1.4% positivity for HBs Ag 9. Biancone et al found a higher prevalence of HBs Ag in patients with CD than those diagnosed with UC (10), so was the case in our study. A French study reported positivity of anti-HBc in 2.78% in CD and 1.59% in UC 11.
The prevalence of anti HCV Ab in patients followed for IBD varies from 0.51% to 17.1% 12-14. Longo et al found a higher prevalence of anti HCV Ab in IBD patients than in a control group (5.98% in IBD vs 0.86% in the control group) 15. In our study, the HCV prevalence is higher in IBD patients than general population. HCV infection in Tunisia among IBD patients may be due to these factors: endoscopic procedures and scarifications. In our study no risk factor was identified in comparing positive and HBV/HCV patients. There was no significant difference concerning patient gender, type or the duration of IBD evolution and the different hepatitis risk factors (history of surgery related or not to IBD, dental care, scarification, tattoos, transfusions, unprotected sex).
Hepatitis B reactivation is not uncommon. It can occur either spontaneously or under immunosuppressive therapy (chemotherapy, corticosteroids, monoclonal antibodies...).
Reactivation of HBV can occur in patients with active hepatitis B infection, chronic or past HBV infection. It is commonly discovered in patients receiving chemotherapy for hematological malignancies. Reactivation can also occur in patients receiving bone marrow or stem cell transplantation, receiving chemotherapy for solid tumors, and patients receiving treatment with anti-CD20 antibodies, TNF inhibitors, corticosteroids or other immunosuppressive agents (thiopurines, methotrexate, cyclosporine...) 16.
HBV reactivation includes three phases. During the first phase, alanine aminotransferase (ALT) levels are normal, HBV DNA in the serum is increased and reappearance of HBs Ag in a person who was previously HBs Ag negative or had undetectable serum HBV DNA. In the second phase, ALT levels is increased, symptoms of acute hepatitis may be present. The third phase is a resolving phase 16. The incidence of HBV reactivation is unknown explained by the lack of standardized definitions of the reactivation.
In HBs Ag positive patients, anti HBc Ab (+) and HBV DNA (+), reactivation is defined by an increase in HBV DNA > 2 log10 compared to the baseline rate. In patients with the following serological profile: HBs Ag (+), anti HBc Ab (+) and HBV DNA (-), reactivation is defined as the detection of HBV DNA (> 100 IU / ml). In negative HBs Ag patients (-), anti HBc Ac positive (+) and negative HBV DNA (-): reactivation is defined by reverse seroconversion (HBs Ag (-) to HBs Ag (+)) ± HBV DNA (+) or detection of viral B DNA with HBs Ag negative.
Rituximab (anti-body that targets the B-cell CD20) is commonly prescribed in non-Hodgkin lymphoma, and has been reported to improve the end of treatment. A meta-analysis showed that among HBc Ab (+) or HBs Ag (+) cases series, there was a higher rate of HBV reactivation for patients who received rituximab-based regimen. In HBs Ag positive patients HBV reactivation ranged from 2.17% to 23.8% in HBs Ag (+) 17. HBV reactivation is possible even in case of positive HBs antibodies 17,18.
Hepatitis B reactivation with anti-TNF alpha antibodies has been reported in both chronic and occult hepatitis B. In patients followed for IBD or rheumatoid arthritis, hepatitis B reactivation was estimated at 37% in HBs Ag positive patients, and vary from 0% to 8.1% in patients with isolated anti HBc antibodies 19,20. In patients under azazthioprine, the reactivation risk is very low especially when prescribed on monotherapy. In our study, no cases of reactivation occurred under immunosuppressive therapy.
Antiviral prophylaxis has proven its effectiveness in preventing HBV reactivation in patients under immunosuppressive therapies. In HBs Ag positive patients receiving chemotherapy for breast cancer, the risk of reactivation under antiviral prophylaxis with NUC was 0% versus 28.6% in its absence. The relative risk of reactivation decreases by more than 80% on antiviral prophylaxis 21,22. EASL recommend early introduction of NUC for all HBs Ag positive patients requiring immunosuppressive therapy 23.
While ECCO recommends vaccination in all IBD patients with negative HBV markers, more than the three quarters of the patients were not vaccinated against hepatitis B. It is mandatory to intensify vaccination efforts. Young age was a predictive factor of a good immune response to vaccination. Standard vaccination in IBD patients was effective in only 50% 24.
Hepatitis C reactivation is a rare phenomenon. These findings do not support the systematic HCV screening of IBD patients. Reactivation has been reported under corticosteroid. In a retrospective study in IBD patients, seven patients on corticosteroids presented reactivation with a fatal issue in one case due to hepatic failure 17. In our study two cases of viral C reactivation were reported. The 1st patient was under azathioprine and infliximab and the 2nd patient was under azathioprine.
In the literature some cases of acute hepatitis C on rituximab have been reported 25.
In conclusion, the prevalence of hepatitis B infection in IBD patients under immunosuppressive therapy was similar to the general population, while prevalence of hepatitis C was higher than the national prevalence. Screening for B and C viral infections is mandatory in all IBD patients. Vaccination against hepatitis B is strongly recommended.