INTRODUCTION
The disease caused by the SARS-CoV-2 virus, denominated COVID-19, affects primarily the respiratory system. Patients affected by this disease often present fever, cough and dyspnea as their most common symptoms 1,2. In severe cases, patients may develop pneumonia and associated complications, such as acute respiratory distress syndrome with septic shock and ultimately, death 1.
The SARS-CoV-2 virus causes a systemic disease, producing injury on the heart, liver, pancreas and kidney, moreover immune system dysfunction and lymphocytes alterations have been reported 2-5. The liver is one of the most affected organs by this disease and many studies have reported alteration in the levels of liver biochemistry tests such as aspartate transferase (AST), alanine transferase (ALT), total bilirubin and albumin 6-9. Several degrees of elevation in the level of hepatic enzymes have been reported. For example, alterations of AST and ALT have been reported ranging from 2.5% - 50% and 2.5 - 61.1% of the patients, respectively 10. Moreover, some studies have found an association between liver biomarker levels and severity of the disease. For example, in a cohort of 1099 patients, Guan et al. 11 observed elevated levels of AST in 18.2% of the patients with non-severe disease and in 39.4% with severe disease. Similarly, Huang et al. 2 reported that the proportion of liver damage in intensive care unit (ICU) patients (61.5%) were higher than non-ICU patients (25.0%). In relation to the levels of total bilirubin (TB) these can rise in 0% to 35.3% of the cases 6,8,11.
Hepatic injury caused by SARS-CoV-2 can arise from various mechanisms, such as direct infection of liver cells, systemic inflammation with production of proinflammatory cytokines, hypoxia, microthrombosis and drug-induced liver injury 7,9,10. For example, it has been shown that SARS-CoV-2 can directly infect liver cells, given that angiotensin converting enzyme 2 (ACE2), which is an important trans-membrane receptor for viral entrance to the cell, is expressed in the liver and bile duct cells 12. Data from two independent cohorts revealed ACE2 expression in 2.6% of hepatocytes and 59.7% of cholangiocytes, suggesting that SARS-CoV-2 could directly bind to ACE2-positive cholangiocytes and alter hepatic function 13.
Liver biopsy samples from patients who died from COVID-19 showed moderate microvesicular steatosis, as well as, mild lobular and portal inflammatory activity 10, however, these nonspecific lesions could have been caused by either SARS-CoV-2 or druginduced injury 14. It has been suggested that drugs toxicity may contribute significantly in the alteration of liver biochemistry tests 7. Liver damage can occur after the use of multiple medications, such as antivirals, antibiotics, traditional medicine, antipyretics, and pain relievers 14.
The aim of this study was to identify the prevalence of alterations in liver function tests in Peruvian patients hospitalized for COVID-19. Also to identify association between severity of the disease and elevation of these biomarkers.
MATERIALS AND METHODS
Study design and inclusion criteria:
A descriptive, retrospective and cross-sectional study was performed. Patients admitted to hospitalization wards and intensive care units (ICU) for COVID-19 were enrolled from May 22 to June 11, 2020. The study was performed in 4 public hospitals in Peru: Arzobispo Loayza and Hipolito Unanue from Lima, and Santa Rosa and Cayetano Heredia from Piura. Inclusion and exclusion criteria were considered as follows.
Inclusion criteria:
Patients older than 18 years old hospitalized from May 22 to June 11, 2020 with a diagnosis of COVID-19, according to clinical suspicion and confirmation by serological and/or molecular assays.
Exclusion criteria:
Patients under 18 years of age. Patients older than 18 years hospitalized without a diagnosis of COVID-19 from May 22 to June 11, 2020. Patients over 18 years old who did not have the required data according to the data collection sheet.
Data collected from the patients included information regarding: age, sex, weight, height, general and respiratory symptoms, past medical history, prior medication before hospitalization, laboratory tests and data on hospital stay. The patients enrolled presented to hospitalization with 10-15 days since disease onset. Liver biochemistry studies were obtained on the first day of hospitalization before starting hospital care. This study was approved by the ethics board of the "Hospital Nacional Arzobispo Loayza".
Liver biochemistry tests and reference range
The following laboratory tests and their reference range were included: alanine aminotransferase (ALT) with normal values between 9-40 U/L, aspartate aminotransferase (AST) with normal values between 13-36 U/L, alkaline phosphatase with normal values between 35-129 U/L, total bilirubin with normal values between 0.3-1 mg/dl and albumin with normal values between 3.5-5 g/dL. All laboratory parameters and their reference range were standardized in the four study sites. Altered liver function test was defined as the elevation above the upper limit of normal (ULN) of any of the biomarkers aforementioned. Patients with elevation of alkaline phosphatase underwent an abdominal ultrasound to rule out alterations in the billiary tract, such as choledocolithiasis.
Severity of COVID-19 disease
Patients were classified according to their clinical characteristics and laboratory parameters. Severity of the disease was defined according to Peruvian national guidelines developed by the Ministry of Health 15. For methodological convenience patients were categorized into not severe (mild and moderate cases) and severe.
A severe case was considered a patient with acute respiratory infection with two or more of the following:
Respiratory rate of >22 per minute or arterial partial pressure of CO2 (PaCO2) <32 mmHG. Mental state alteration. Systolic blood pressure of <100 mmHg or mean arterial blood pressure of <65 mmHg. Arterial partial pressure of oxygen (PaO2) <60 mmHg or ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300. Clinical signs of respiratory failure such as: nasal flaring, usage of accessory muscles, among others. Serum lactate >2 mosm/L.
Statistical analysis
The data corresponding to the study variables were compiled in a database using the Excel program. For the descriptive analysis of the qualitative variables, the frequencies and percentages were calculated. In the case of quantitative variables, measures of central tendency were used, such as the mean and standard deviation. To evaluate the differences between the qualitative variables, the Chi Square test was used and for the quantitative variables the Mann-Whitney U test, in both cases statistical significance was considered a value of p <0.05. Odds ratio (OR) were calculated to determine associations between variables and the outcome of interest (abnormal liver function tests). The IBM SPSS v 23 program was used to perform the statistical analysis.
RESULTS
A total of 1,100 patients were enrolled during the study period. The 81.7% (899/1100) of patients who were admitted to hospital with the diagnosis of pneumonia due to COVID-19 had altered liver function tests. Among these, AST and ALT were altered at admission in 64.7% and 63.7%, of the patients respectively. The majority of our patients with elevated liver enzymes were registered in the age group ≥60 years old (46.8%). Also we could highlight that in the age group 20-39 years old the proportion of patients with elevated liver enzymes was significantly higher than the group with normal transaminases (Table 1). When comparing gender, it was evidenced that 70.6% of male patients had altered liver function tests compared to 29.4% of female patients. Moreover, female patients were less likely to present altered liver tests OR=0.53 (95% CI: 0.39-0.73; p<0.01).
Table 1 Demographic characteristics of 1100 patients admitted for COVID-19 and their association with liver function tests.
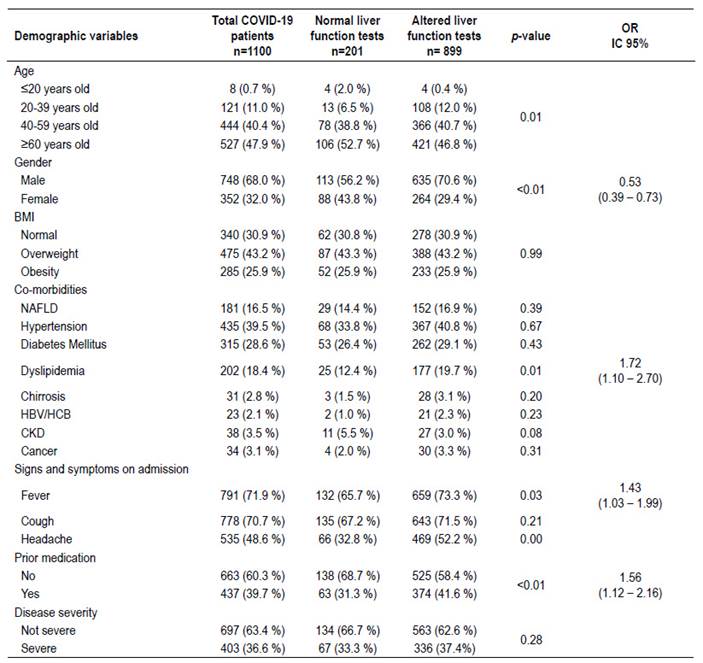
On the other hand, past medical history, co-morbidities and body mass index (BMI) were evaluated in relation to liver function tests. Patients with BMI higher than 25 kg/m2 did not show a significant elevation in the liver function tests compared to patients with BMI lower than 25 kg/m2. Other factors associated with elevated liver enzymes were: dyslipidemia OR=1.72 (95% CI: 1.102.70; p=0.01) and previous medication OR=1.56 (95% CI: 1.12-2.16, p<0.01). In the case of signs and symptoms on admission, it was evidenced that patients with fever OR=1.43 (95% CI: 1.03-1.199, p=0.03) were more likely to present altered liver function tests. Interestingly, patients with non-alcoholic fatty liver disease (NAFLD) did not show association with alteration in liver function tests.
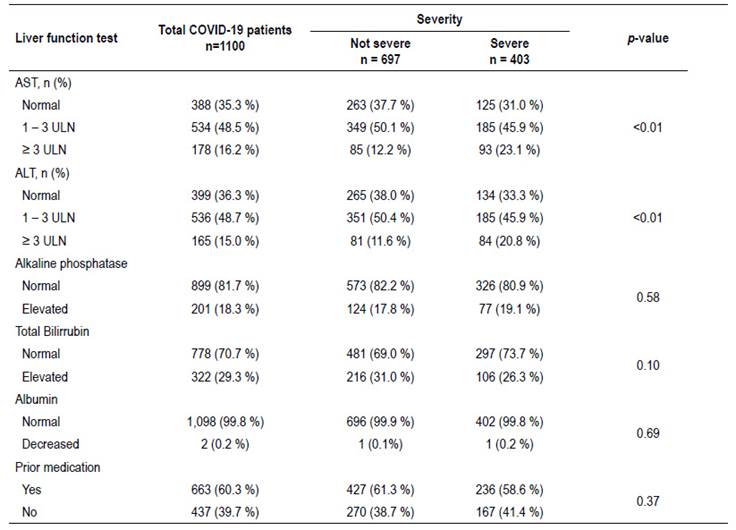
Table 2 shows the association between each liver function test evaluated and the severity of the disease. It was evidenced that levels of AST and ALT <3ULN did not show significant association with disease severity. On the other hand levels of ≥3ULN were significantly associated with greater severity of the disease. Meanwhile, alkaline phosphatase, total bilirubin and total albumin did not show significant differences.
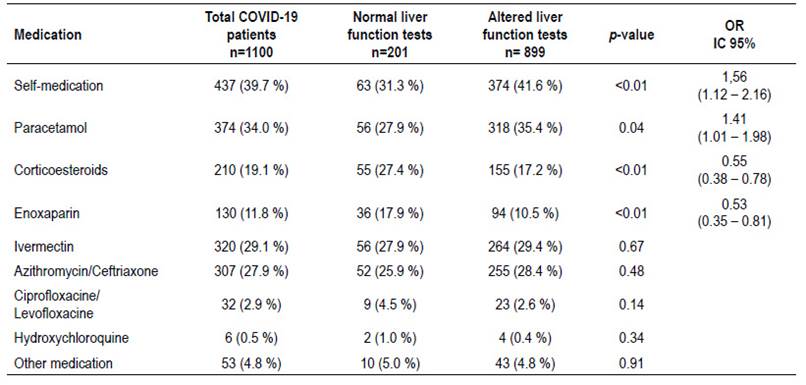
Table 3 shows the association between selfmedication taken before admission and alteration in liver function tests. We could highlight that 437 patients (39.7%) took self-medication before admission and among these, 63 patients had normal liver function tests and 374 had some degree of alteration. Patients who took self-medication had significantly higher alteration in hepatic function tests, also, self-medication was associated with an OR=1.56 (95% CI: 1.122.16; p<0.01). The most frequently consumed drugs were paracetamol (34.0%), ivermectin (29.1%) and azythromicin/ceftriaxone (27.9%). Patients who took paracetamol had a higher risk to develop alteration in hepatic function with an OR= 1.41 (95% CI: 1.01-1.98; p=0.04). Meanwhile, in patients who took ivermectin and azythromicin/ceftriaxone did not show significant differences among groups. On the other hand, patients taking corticosteroids OR=0.55 (95% CI: 0.38-0.78; p<0.01) and enoxaparin OR=0.53 (95% CI: 0.350.81; p<0.01) showed a significant lower proportion of alteration in hepatic function tests.
DISCUSSION
COVID-19 is a heterogeneus and complex disease, although it mainly affects the respiratory system, there is growing evidence that it can cause injury on other organs. The gastrointestinal system, particularly the liver, are affected by this disease in variable degrees 16. Liver dysfunction in the context of COVID-19 may represent an important prognostic variable, therefore the assessment of the liver involvement is essential in patients care. For example, it has been found that increased levels of AST, ALT and AST/ALT ratio are important markers of mortality risk, severity of disease and probability of ICU admission 17-19.
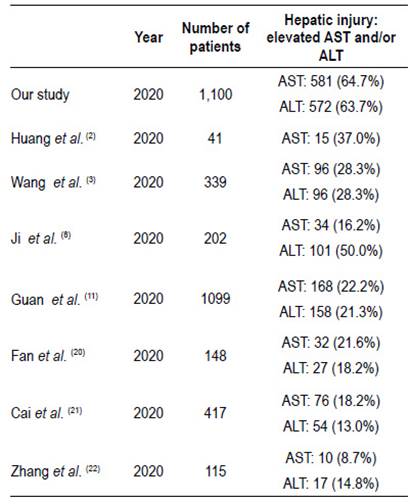
We performed the largest study evaluating the liver function tests of patients admitted with a diagnosis of COVID-19 in Peru, with 1,100 patients enrolled during the study period. A high prevalence (81.7%) of the patients presented at least one altered liver biochemistry test. Moreover, AST and ALT were altered at admission in 64.7% and 63.7%, of the patients respectively. These findings are significantly higher than those reported in previous literature with almost 3 times higher compared to others studies, as shown in Table 4. One of the largest series of patients was performed by Guan et al. 11, evaluating 1,099 COVID-19 patients in China and among these, 21.3% and 22.2% had elevation of AST and ALT, respectively. We could highlight that this study uses the value of 40 U/L as the upper normal limit for both enzymes, which is similar to our study. On the other hand other studies 20-22 have used values above 1.5 to 2 times greater the ULN to determine liver tests abnormalities and reported alterations ranging from 13% to 21.6%. It is important to consider that aforementioned studies did not evaluate important variables such as self-medication. One possibility that may explain the high frequency of alteration in liver function tests could be related to the high proportion of patients who took self-medication.
When evaluating the demographic variables in relation to hepatic injury, it was evidenced that some factors such as age, gender, co-morbidities and use of previous medication were associated. Previous evidence has shown that patients older than 50 years are those who are more severely affected by the disease 23. Similar to our study, in which we report that patients older than 60 years old had a greater prevalence of alteration in liver enzymes. Remarkably, we report that patients in the 20-39 years old group had also a significant higher alteration in liver function tests, which have not been reported previously. Regarding gender, it was evidenced that male patients had elevated liver tests (70.6%) compared to of female patients (29.4%), this difference was statistically significant. Moreover, female were significantly less likely to present abnormal liver function tests.This finding is similar to what has been reported in previous literature, proposing that men are more likely to be affected by the disease and present higher degrees of liver injury 23,24. Also, the majority of the patients hospitalized in our study were male (68%).
Several co-morbidities have been associated with a poor prognosis, greater severity and mortality in patients affected by COVID-19. Diseases like diabetes mellitus, obesity and cardiovascular diseases are among the most important 24. Moreover, patients with chronic liver diseases may also be at a higher risk of developing serious complications 25. In the current study, no clear association was found between the alteration of liver function tests and co-morbidities like hypertension, diabetes mellitus, chronic liver diseases and obesity. We could highlight a low prevalence of chronic liver diseases in our study, such as cirrhosis (2.8%) and viral hepatitis B/C (2.1%), this finding is in agreement with a previous meta-analysis, showing that COVID-19 patients present a global prevalence of chronic liver diseases of 2.8% 26. In the case of Peru, the leading causes of chronic liver diseases are non-alcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH), followed by alcholrelated liver disease and viral hepatitis. For example, a study of 200 transplants performed in Peru, reported that the most frequent indication for this procedure was NAFLD with 35% 27. Another study reported the most common causes of chirrosis which included: alcohol (28%), NAFLD (21.3%), Hepatitis B virus (15.2%) and Hepatitis C virus (11.8%) 28. These findings highlight the need to maintain a careful observation on patients with liver diseases and consider them high-risk patients 26.
In the case of past medical history, we found that dyslipidemia, fever on admission and taking medication prior to enrollment were significantly associated with abnormal liver tests. These findings could be explained by a variety of reasons. Firstly, alteration in lipids may predispose patients to hepatic steatosis and NAFLD, making them more susceptible to liver damage 8. Nonetheless we observed that patients with NAFLD in the current study did not show signifant differences in the level of transaminases. This could be explained because NAFLD and even NASH do not neccesarily present with elevation of liver enzymes, in fact many patients with hepatic fibrosis may still have normal transminases levels 29,30. Moreover, taking previous medication can cause variable degrees of elevation in hepatic enzymes, depending on the pharmacologic agent and doses. In the case of fever, this represents a first line response from the body to combat infection, and could be related to the degree of systemic inflammation, which may induce liver injury 7,16.
An evaluation of disease severity and its association with each individual liver biochemistry test was performed. The levels of AST and ALT were significantly associated with disease severity (p<0.01), particularly levels 3 times greater than the upper limit value. On the other hand, other tests like albumin, alkaline phosphatase and total bilirubin did not show significant differences. A previous review, stratified clinical variables in three levels of risk for developing severe disease, among hepatic function tests it was evidenced that hypoalbuminemia was classified in the highest risk group, meanwhile AST and ALT in the lowest risk group 23. However, another systematic review revealed that AST, ALT and total bilirubin are strongly correlated with disease severity 31. In our study we could also confirm that elevations of AST and ALT three times above the upper limit of normal were associated with disease severity. It is of great importance to mention that only 81/1,100 (7.4%) patients were admitted to ICU in our study, which could explain that minimal rates of hypoalbuminemia found. According to previous literature, this finding is often associated with long hospital stays and tends to appear as a late finding, particularly in patients on ICU 16. Most of our patients were in regular hospitalization wards and albumin assessment was performed on admission.
COVID-19 may induce liver injury and dysfunction by several mechanisms including: direct cytotoxicity of viral replication, systemic inflammatory response, hypoxia-mediated injury, microthrombi formation, drug-induced injury and reactivation of previous liver diseases 7,10. Firstly, liver injury caused directly by SARSCoV-2 may be one of the most important mechanisms, given that ACE2 receptors are expressed in many human tissues apart from the lungs. Previous reports have observed the abundant expression of this receptor in enterocytes and cholangiocytes, and in a lesser degree in hepatocytes 12,13,32. These findings may explain direct viral invasion on these cells, causing elevation in liver biochemistries. Although, cholangiocytes are most likely to be affected by the virus 12,13, the dysfunction of these cells may cause indirect transaminases elevation. However, alkaline phosphatase, a marker of billiary tract affection, was only elevated in 18% of our patients, with no significant differences in severity. Also, levels of total bilirubin, another marker of cholestasis may be elevated in 0% to 35.3% of the cases. In the current study, we found an elevation of bilirubin in 29.3% of the patients, with no significant differences in severity. Altogether, these findings may indicate a hepatocellular pattern rather than a cholestasic pattern, in patients affected by COVID-19 9,10,17.
Finally, given that drug-induced liver injury is an important mechanism for hepatic injury as shown in previous studies 14, an evaluation on medications taken prior to admission was performed. We could observe that 41.6% of the patients with elevated liver enzymes took self-medication before enrollment. It was evidenced that self-medication was associated with elevated liver enzyme with an OR=1.56 (95% CI: 1.12-2.16; p<0.01). Patients who took paracetamol had a higher risk to develop alteration in hepatic function with an OR= 1.41 (95% CI: 1.01-1.98; p=0.04). Given that a high proportion of patients took self-medication under no medical supervision, it is possible that they have taken inappropriate doses of paracetamol, considering that consumption of paracetamol at therapeutic doses is unlikely to cause an important liver injury 33-35.
In our study, a variety of other medications were taken by the patients such as Azithromycin/Ceftriaxone, Ciprofloxacin/Levofloxacin, Hydroxychloroquine, Corticosteroids, Enoxapaparin and Ivermectin. We could highlight that consumption of corticosteroids OR=0.55 (95% CI: 0.38-0.78; p<0.01) and enoxaparin OR=0.53 (95% CI: 0.35-0.81; p<0.01) may be protective factors against liver injury. Possible mechanisms that may explain this finding is that corticosteroids could diminish systemic inflammation, avoiding liver involvement by cytokines 29,36. On the other hand, the administration of anticoagulants such as enoxaparin, may have contributed to prevent thrombi formation, which could in turn generate hypoxia mediated liver injury, as reported previously37,38 . Widely used medications like Hydroxychloroquine and Ivermectin did not show association with alteration in liver function tests in our study. However, previous studies have shown that they may cause liver injury and should be avoided as they do not provide a clear benefit 34,36.
These findings highlight the high proportion of patients that take self-medication without medical supervision, which may contribute to later liver dysfunction. Moreover, future studies are required to determine the protective role of corticosteroids and enoxaparin and to examine the proposed mechanisms.
In conclusion we found that 81.7% of patients hospitalized for COVID-19 had alteration in liver function tests. Risk factors for liver injury were being male, history of dyslipidemia, fever on admission and taking prior medication. Severity of the liver injury by COVID-19 was associated with values of AST and ALT three times above the limit level of normality. It was found that patients on self-medication and taking paracetamol were more likely to present liver injury. Finally, corticosteroids and enoxaparin may have a protective role against liver dysfunction in COVID-19 patients. Further studies are required to evaluate alteration in liver function tests as prognostic predictors.