INTRODUCTION
Laparoscopic approach in rectal cancer has showed good shortand long-term outcomes by various randomized and controlled studies in recent years.
Equivalent surgical and perioperative outcomes with oncological similarities to open surgery are known 1, even for locally advanced tumors 2. The most representative trials 3,4 compared surgical specimen quality, complication, and leakage rates, as well as overall survival and disease-free survival with no difference between both approaches 5,6. Laparoscopic technique for middle and lower rectal cancer after neoadjuvant treatment was also corroborated by the Korean trial 7,8, however, other trials failed to demonstrate non-inferiority, not recommending the use of laparoscopic surgery as the standard for rectal cancer 9,10.
In Latin America, colorectal cancer ranks third in incidence among other cancers, however, the estimated number of new cases of rectal cancer per thousand inhabitants in Peru will double by 2040 11. Open surgery is the standard surgical treatment in the public health system for this type of cancer and only some specialized centers perform minimally invasive surgery (MIS) in our country. The pelvic approach is difficult enough due to its anatomical location and requires a longer learning curve 12, however, the sum of the narrowness of the pelvis in patients from the Andean region due to their short stature and the prevalence of locally advanced tumors because of health screening programs deficiencies, increases its difficulty and makes it challenging.
The main aim was to describe the experience in our population and compare the perioperative results between open and minimally invasive surgery in public health hospital with the following parameters: surgical specimen and mesorectum quality, circumferential resection margin (CRM) and distal margin (DM), tumorfree surgical margins, hospital stay and postoperative complications. Recurrence-free survival (RFS) and overall survival (OS) between both approaches were described. Propensity score matched (PSM) was used.
METHODS AND MATERIALS
Patients and data collection
A retrospective cohort study was conducted in a single center. Patients admitted at Instituto Nacional de Enfermedades Neoplásicas (INEN), Lima, Perú, between January 2016 and December 2020 for elective sphincter-sparing surgery with rectal cancer diagnosis were included. All patients who had open elective surgery or minimally invasive surgery through laparoscopy (LapTME), or combined laparoscopy with transanal approach (TaTME) were included. Patients who presented a biopsy of adenocarcinoma of the rectum reviewed at our institution, non-metastatic rectal cancer, and patients with a known date of surgery and postoperative control up to at least 30 days after surgery were eligible for analysis. Patients with sigmoid colon cancer or tumors above 15 cm from the anal margin by colonoscopy, clinical stage IV rectal cancer, abdominoperineal resections, synchronous and/or new cancer three years after diagnosis, patients who required multiorganic resection (two organs) and patients with incomplete medical history or missing data were excluded. Data was reported in line with STROCSS 2019 criteria 13 and with ethical approval from Institutional Review Board in accordance with the Declaration of Helsinki.
Demographic, clinic, and pathological characteristics were obtained and length of postoperative hospital stay, postoperative complications and recurrence-free survival (RFS) and overall survival (OS) were measured.
Conceptual definitions
Patients with middle and lower rectal cancer stage III received neoadjuvant treatment with oral capecitabine 825 mg / mts2 concurrent with radiation therapy of 50.4 cGys in 28 sessions. A positive CRM was considered a margin of 1 mm or less, a positive DM of less than 1 mm from the tumor, and an optimal quality of the mesorectum according to the Quirke P et al. classification of complete or near complete mesorectum 14. A major complication defined as grade III or more of Dindo-Clavien classification 15. The recurrence diagnosis with pathology through surgery or biopsy, or lesions of recent appearance demonstrated radiologically was registered. Loco regional recurrence was defined as any recurrence at the level of the pelvis or pelvic lymph nodes and systemic recurrence as any recurrence outside the pelvis. The time from the date of surgery to the date of recurrence, death or date for the last control at study closure was measured for recurrence-free survival time, and the date of surgery to the date of death or study closure date for the overall survival. Every 3 months for physical examinations and 6 months for radiological tests and proctoscopy was made for the follow-up.
Statistical methods
The analysis was used under the modality of propensity score matched with six covariates to reduce the bias between both groups, age, sex, treatment with neoadjuvant chemo-radiotherapy, tumor size, location of the tumor in the rectum and infiltration grade (pT), with a standardized mean difference tolerance of less than 0.2 16,17. The qualitative variables were presented in numbers, percentages, and quantitative variables with median and interquartile range (IQR) or mean with standard deviation according to their distribution. The statistical analysis was carried out through the R program version 4.04 and Rstudio version 1.9.0. The comparison between variables was with the Fisher test and the Mann-Whitney U test, respectively, and a significance was set at p-value <0.05. Survival curves were estimated with the Kaplan-Meier method and the log-rank test to contrast them.
RESULTS
Patients and clinical characteristics
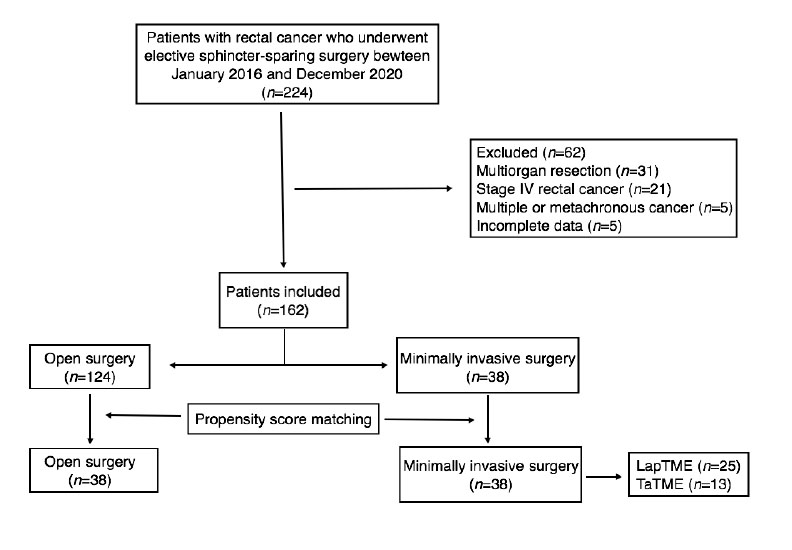
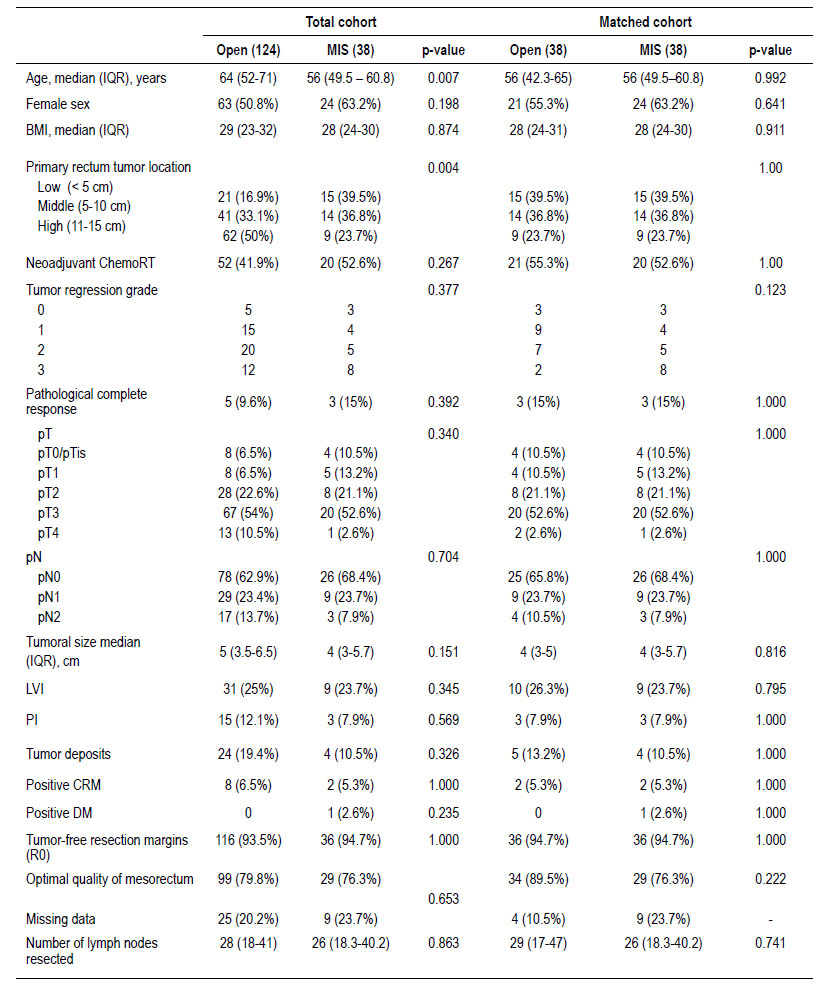
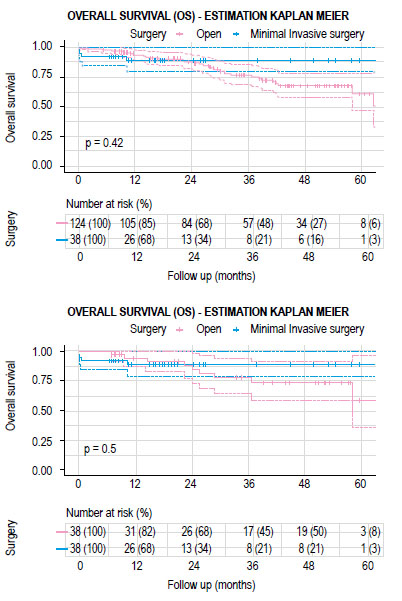
There were 162 cases included in the final analysis with an average follow-up time of 31.5 months. Open surgery group had 124 cases and 38 cases of MIS, after matching 38 cases were compare (Figure 1). Table 1 describes the clinical characteristics of the analyzed population. LapTME was performed in 25 cases and 13 cases had TaTME surgery. Older patients (64 vs. 56 years, p=0.007) and high rectum tumors (50%) were found frequently in the group of patients who had open surgery (p=0.004) before matching. The rate of female patients (63.2%) and the use of neoadjuvant chemo radiotherapy was higher in the group with MIS (52.6% vs 41.9%) but without significant differences between groups. After matching with the propensity score, all variables were balanced.
Pathological characteristics
The pathological characteristics are described in Table 1. The pT, pN, tumor size, presence of lymphovascular invasion, perineural invasion, tumoral deposits, and the tumor regression grade of the patients who received neoadjuvant treatment did not show differences between both groups. The quality of the surgical specimen and the tumor-free resection margins also showed no differences. Positive CRM was present in 5.3% after matching between both groups (p=1.000). The optimal quality of the mesorectum (89.5% vs 76.3%, p=0.222) and the number of lymph nodes resected was slightly higher in the open surgery group (p=0.741).
Perioperative outcomes
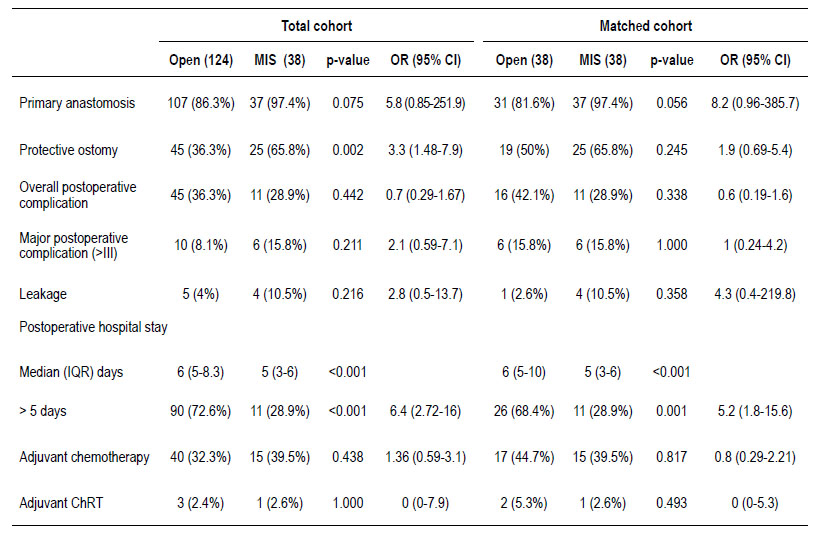
Table 2 shows the surgical and postoperative outcomes. A protective ostomy was mainly performed in the MIS group (65.8% vs. 36.3%, p=0.002) with an OR 3.3 (95% CI; 1.48-7.9), but was then balanced after the matching (p=0.245). The overall postoperative complication rate was slightly higher in the open surgery group. On the other hand, major complication rate was 15.8% and 10.5% of leakage was reported in the MIS group without significant difference between groups. Postoperative hospital length stay longer than 5 days was frequently seen in the open surgery group (72.6% vs 28.9%, p=0.001) with an OR 6.4 (95% CI; 2.72-16), even after matching (68.4 % vs 28.9%, p=0.001) with an OR 5.2 (95% CI; 1.8-15.6). There was no difference between the groups in relation to the use of adjuvant treatment.
Long term outcomes
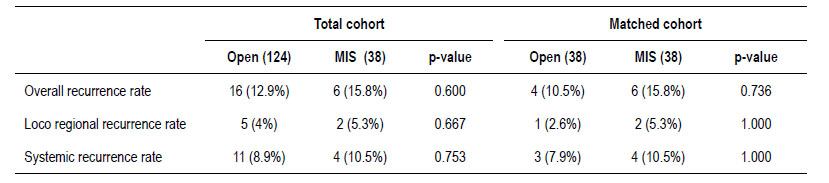
There were no significant differences in the recurrence rate according to each surgical approach before and after matching showed in Table 3. In the total cohort, there were 16 cases (12.9%) of recurrence in the open surgery group and 6 cases (15.8%) in the MIS group (p=0.600). Eleven cases (8.9%) with distant recurrence were reported in open surgery (5 cases with lung, 3 with liver and lung, 1 with liver, 1 with lung and brain, and 1 with retroperitoneal lymph node and lung metastasis) and 4 cases (10.5%) in MIS group (2 cases with liver and lung and 2 cases with liver metastasis) (p=0.753). In addition, 5 cases (4%) had loco regional recurrence in the open surgery group (3 cases at the level of the anastomosis and 2 cases in the pelvis) and 2 cases (5.3%) with pelvic recurrence in MIS group (p=0.667).
The mean follow-up time for the group of patients who had open surgery was 34.2 months (IQR 20.3-50.3 months) and 22.7 months (IQR 10.3-26.2 months) for the MIS group. There were no significant differences between both groups in recurrence-free survival (RFS) and overall survival (OS) (Figures 2 and 3). The estimated recurrence-free survival at 3 years in open surgery and MIS was 71.8% (95% CI; 0.58-0.89) and 70% (95% CI; 0.56-0.88) (p=0.4) and the estimated OS at 3 years was 77.7% (95% CI; 0.64-0.94) and 88.9% (95% CI; 0.79-0.99) (p=0.5) each.
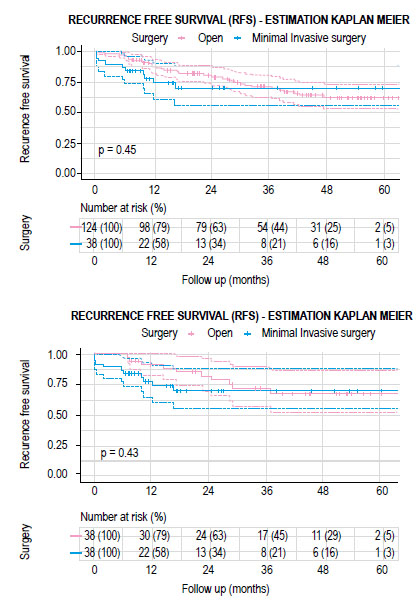
Figure 2 Kaplan-Meier curves showing recurrence free survival according to open or minimally invasive surgery. A) total cohort and B) matched cohort.
DISCUSSION
Minimally invasive surgery for rectal cancer includes laparoscopy, TaTME, NOTES and robotic surgery, commonly practiced in many countries with satisfactory oncological results 18; however, there is not enough evidence in the Andean region of South America that support MIS as standard treatment for rectal cancer. There is only one comparative study between laparoscopic and open surgery performed in Chile that showed similarity between both techniques with promising results 19. Our study represents the first analysis in the Peruvian population comparing shortand long-term outcomes through clinical pathological, surgical, and survival results in the Andean region of the continent. The clinical characteristics of the patients were balanced with six covariates through the PSM to standardize the comparative groups as recorded in other studies 20.
Short-term results were measured through the number of harvested lymph nodes, the quality of the mesorectum in the surgical specimen, and the positive circumferential resection margin and distal margin, with no difference shown between both groups. The positive CRM was present at the same rate as other international trials and even lower in some cases 4, probably due to the 2 mm cut-off point considered for positive CRM in the European trial, compared to the rest of trials which used 1 mm 9,10. On the other hand, the Korean trial showed the lowest rate of positive CRM in middle and lower rectal cancer where all received neoadjuvant treatment with different chemotherapy regimens which could contribute in the sterilization of the margins in the final specimen and because a better selection of patients with an unthreatened CRM (>1 mm) by preoperative magnetic resonance imaging. Although, a low body mass index was reported which could decrease the complexity of pelvic approach only by LapTME (7). No incomplete mesorectum excision of the surgical specimen was reported in our study despite the complexity of the dissection of the narrow and deep pelvis. Optimal mesorectum quality rate was recorded as complete and almost complete excision comparable between both groups.
Overall, postoperative complications between open surgery and MIS in rectal cancer are similar worldwide, however the rates are different according to the geographical location of each trial. In our experience, the major complication and leakage rates did not show differences between groups group in comparison with other trials 4,9, although, a higher rate was recorded in the MIS. The relationship between multiple stapling during the resection of the rectum in the pelvis and the proximity of the lesion to the anal canal 21 could be the reason. Promising results with the use of robotic surgery were expected, but a trial failed to show a decrease in difficulty with similar conversion and complication rates compared to laparoscopic surgery in lower rectal surgery with interesphincteric resection (22). On the other hand, TaTME surgery, described as a new solution for and old problem, decreased the conversion rate but recorded slightly higher leakage rate 23,24. Intersphincteric resections, very low anastomosis, the use of neoadjuvant chemo radiotherapy, and the large tumor size are all risk factors described for leakage 21,25, and were present in our study. In South America, the study in Chile showed a higher leakage rate in laparoscopic surgery alone [19], while the Brazilian study reported a low rate with major complications, probably due to the fact that most of their patients had colonic resections and benign lesions 26. The risk of anastomotic failure in rectal surgery is known to be higher than in colon surgery and requires more training and experience over time 3.
Postoperative hospital stay showed a great difference between groups with earlier discharge in the MIS group. Patients who had minimally invasive surgery for rectal cancer had faster recovery and discharge without increasing the risk of postoperative complications or readmissions. The hospital length stay in our study for MIS surgery was shorter than the aforementioned studies (3,4) because of the routine use of the enhanced recovery after surgery program applied at our institution and because of the high number of patients with ostomies, even though just a few randomized trials described it 10.
Non-inferiority survival outcome between laparoscopic and open surgery in rectal cancer has been described by multicenter randomized trials 6,8, however, others concluded the opposite 27. Some other trials had not been able to reach the gap imposed because of surgical specimen quality 9,10 despite the long term analysis showed no differences in the disease-free survival and local recurrence (28,29). In our study, the analysis did not showed differences between both groups, with overall recurrence rates of 15.8% with 10.5% of systemic and 5.3% of loco regional recurrence in the MIS group, with an estimated probability of survival at 3 years of up to 71%. Other studies had the same loco regional recurrence rate in the laparoscopic surgery group but with better recurrence free survival rate 6,28. In the Latin American population, a higher rate of systemic recurrence has been described 19, however, the difference between
RFS could be related to the follow-up time or the doses used in neoadjuvant treatment for stages II and III 28,29, adjuvant chemotherapy and its level of adherence to treatment, which was not measured in our study.
The limitation of our comparative study was the retrospective cohort design; however, the use of the PSM with the six clinical variables significantly reduced the biases in the results on pathology, perioperative and survival outcomes. There is also a weakness to statistical power due to the reduced sample analyzed after matching. A larger sample and more studies that demonstrate shortand long-term outcomes between both surgical techniques are required to promote the standard use of MIS for rectal cancer in Peru.
In conclusion, shorter postoperative hospital stay with no increase in the major complication rate in the MIS group was reported. The recurrence-free survival, overall survival, and recurrence rates were similar compared with open surgery. MIS is as safe as open surgery in nonmetastatic rectal cancer at a referral center in Peru in the public health system and could be recommended as a standard approach in selected patients.