INTRODUCTION
Drug induced liver injury (DILI) can be can be triggered by many medications including herbal medicines and dietary supplements 1. Antituberculosis drugs can cause DILI in many clinical presentations ranging from asymptomatic forms, drug reaction with eosinophilia and systemic symptoms (DRESS) and acute liver failure (ALF) following to death in some cases 2.
We present the case of a 37-year-old woman with a smear- positive pulmonary tuberculosis who started treatment with first-line regimen of antituberculosis drugs and 4 weeks later presented jaundice, somnolence and a morbilliform generalized rash with progressive neurologic deterioration which had a fatal outcome.
CASE REPORT
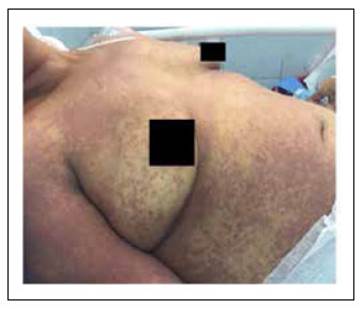
A 37-year-old woman with a smear-positive pulmonary tuberculosis, started treatment with the first-line regimen consisting on Isoniazid 5 mg/kg, Rifampicin 10 mg/kg, Ethambutol 15 mg/kg and Pyrazinamide 25 mg/kg. On the third week of treatment, she presented general malaise, right upper quadrant abdominal pain and fever. A week later, she developed generalized jaundice, somnolence and a morbilliform rash that began on the chest and upper limbs and spread through the entire body (Figure 1). On admission, the patient was tachycardic (110/min), febrile (38.6°C), hypotensive (86/60 mmHg), somnolent (Glasgow Coma Scale 14/15) and presented right upper quadrant abdominal tenderness with a hepatic span of 16 centimeters.
Laboratory exams were abnormal as follows: leukocytes 20,400 x 109/L (eosinophils 1,500 x 109/L), platelets 246,000 x 109/L, glucose 36 mg/dL, prothrombin time of 76 s ( INR 5.98), AST 2553 U/L, ALT 1,635 U/L, alkaline phosphatase 161 U/L and total bilirubin 8.1 mg/dL with a direct predominance of 6.4 mg/dL. Serological tests for HIV, HAV, HBV, HCV, were all negative. Abdominal ultrasound revealed mild hepatomegaly with no focal liver lesions.
DRESS syndrome and ALF due to antituberculosis drugs was highly suspected. Initial general measurements were admission to intensive care unit, suspension of the drugs, corticoids, antihistamines and N-acetylcysteine administration. On the third day of hospitalization, the neurological condition worsened and the patient had a fatal outcome.
DISCUSSION
DILI is caused by intrinsic dose-dependent or idiosyncratic reactions; by immune reaction or by a metabolite, with latency periods ranging from days to months. Our case corresponds to an idiosyncratic reaction 1.
It is considered that an antituberculosis drug induces liver injury if it meets one of the following criteria: 1) Aminotransferases > 3 times upper limit of normal (ULN) and alkaline phosphatase > 2 times, without symptoms; 2) Aminotransferases > 5 times ULN, with or without symptoms; and 3) Bilirubin >2 times the normal value 1-3. In our case, there was hepatocellular involvement with a Ratio (R) > 10 and aminotransferase levels > 20 times the ULN.
Likewise, our patient meets the criteria for severe DRESS syndrome: cutaneous rash, eosinophilia and systemic involvement with liver compromise 4. It is usually difficult to establish its relationship with a certain drug and, therefore, its probability is objectified using the European Registry System RegiSCAR (severe cutaneous adverse reaction). The patient obtained 5 points for which she was classified as a probable case, having also ruled out other causes of acute liver failure 5.
Antituberculosis drugs can cause DRESS syndrome in 1.2% of patients receiving these medicines. In a 4-year retrospective study, Ethambutol was associated with the majority of the events in up to 53.5%, followed by Rifampicin with 26.7% and isoniazid with 6.7% 6. On the other hand, ALF can occur in 35% of patients with DILI with an overall mortality of 9.7%. Most of these patients developed DILI within the first month of treatment and the ALF occurred in the first 2 months 7.
Early recognition of the syndrome and withdrawal of the precipitating drug is essential. In most cases is reversible but some can progress to a fatal outcome. Is very difficult to identify the responsible drug because all of them can produce this clinical picture. In ALF due to anti-tubercular drugs unresponsive to medical treatment, liver transplantation is the treatment of choice. Bartoletti et al described two successful cases of liver transplantation during anti tuberculous treatment that prevented progressive complications in a 14-year-old boy and a 50-year-old woman with pulmonary tuberculosis 8.
In our country, liver transplantation is not yet a universal health practice, which is why our patient could not access to this option. Given this, it is essential to strengthen pharmacovigilance for early detection and early management.