INTRODUCTION
Acid peptic disorders are among the most prevalent gastrointestinal diseases. Only in 2014, there were over 5.6 million outpatient visits in the United States for gastroesophageal reflux disease and reflux esophagitis 1. As a result, gastric-acid suppressants (GASs), which include histamine 2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs), are one of the most frequent drug classes used in both primary and specialized health care around the world 2,3. Between 2009 and 2015, approximately 600 million patients in ambulatory medical care clinics in the United States had documented GASs use 4. Since their release in the 1980s, PPIs have taken the place of H2RAs as first-line GASs 5. Currently, this drug set is among the twenty most prescribed medications at office visits in the United States, and it is estimated that about 113 million PPI prescriptions are written every year in this country 6,7. This fact can be explained by several factors, including their therapeutic superiority over H2RAs, their relatively safe side effect profile, good tolerance, and their over-the-counter access in many countries 2,8.
A situation that has gained relevance over the past two decades is the overprescribing of GASs, especially PPIs, due to the potential adverse effects, as well as the increase in healthcare costs 9. Several studies have shown an association between chronic use of PPIs and serious adverse effects, including Clostridium difficile and other enteric infections, intestinal colonization with multidrug-resistant microorganisms, hospital and community-acquired pneumonia, dementia, osteoporotic fractures, hypomagnesemia, and acute interstitial nephritis 4,8,10,11. Recent retrospective observational studies suggest a likely association between long-term, high-dose PPIs use and a low increased risk of first-time ischemic stroke, especially in elderly patients 12,13. In terms of economic burden, GASs prescription leads to a progressive increase of public health costs. PPI prescriptions are estimated to cost approximately $10 billion per year in the United States 14,15. Only in 2010, income from the sale of esomeprazole reached $13.6 billion 16. While in the United Kingdom, spending on PPIs reached £430 million in 2004 17. Furthermore, it is calculated that nearly £2 billion is spent unnecessarily worldwide each year due to PPIs prescriptions 18,19.
For all the above reasons, interest in the overuse of GASs has increased in recent years, and a growing number of studies have been carried out worldwide. However, little research has been conducted on the inappropriate prescribing of GASs in Latin America. This study is aimed to assess the appropriateness of GASs use among patients at an internal medicine service of a tertiary-level care hospital in Venezuela.
MATERIALS AND METHODS
Ethics
This study followed the Declaration of Helsinki and was approved by the ethical review board of Escuela de Ciencias de la Salud "Francisco Battistini Casalta", Universidad de Oriente.
Study design
We performed a retrospective record review of patients who were admitted from January 2020 to February 2021 at the internal medicine service of Hospital Universitario "Ruiz y Páez" (HURP), Ciudad Bolívar, Venezuela. HURP is a public academic tertiary healthcare center. Patients were included in the study only once, and their subsequent readmissions were not counted. Medical records of patients whose GASs use prior to admission continued during hospitalization, age <18 years, incomplete data, critically ill patients at the time of admission, or patients requiring intensive care therapy during hospitalization were all exclusion criteria. Sample size estimation was performed by using G*Power 3.0 software 20,21.
Study definitions
Appropriateness of GASs prescription was defined by identifying whether patients had received such drugs according to the indications stated by the United States Food and Drug Administration (FDA), National Institute for Clinical Excellence (NICE) in the United Kingdom, the American College of Gastroenterology (ACG) and the American Gastroenterological Association (AGA) 7,22,23. These approved indications are summarized in Table 1. The prescribing was grouped into two categories, appropriate or inappropriate, in accordance with the previously mentioned indications.
Table 1 Approved indications of gastric-acid suppressants according to FDA, NICE, ACG and AGA .
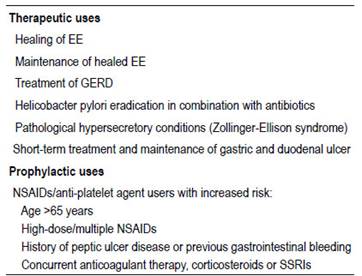
FDA, Food and Drug Administration; NICE, National Institute for Clinical Excellence; ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; EE, erosive esophagitis; GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti-inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors.
Data collection
Data were obtained from paper medical records. Information collected included demographic data, reason for admission, indication for the use of GASs, type of GASs used, route of administration, duration of treatment and their continuation at discharge.
Statistical analysis
In case of normal distribution, continuous variables were expressed as mean ± standard deviation (SD) while were expressed as the median and interquartile range in nonnormal distribution. Categorical variables as frequencies and proportions. Statistical analyzes were calculated by using IBM® SPSS® Statistics 21.0
RESULTS
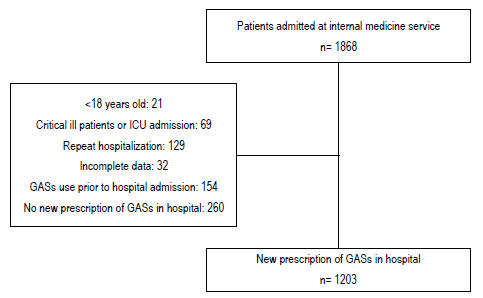
Among the 1868 patients admitted at the internal medicine service of HURP throughout the study period, 1203 patients (64.4%) met the inclusion criteria, as shown in the flowchart (Fig. 1). Six hundred fifty-two (54.2%) were male. The mean age was 54.9 ±17.9 years. The most frequent admitting diagnosis was infectious diseases (19.2%), followed by cardiovascular diseases (18.5%). PPIs were the most frequently prescribed GAS in hospital (98.7%), with omeprazole being the most widely used (87.5%). The main route of administration of GASs was intravenous. The median of duration of gastric-acid suppression therapy in hospital was 7 days (Table 2).
Table 2 Patient demographics and clinical characteristics.
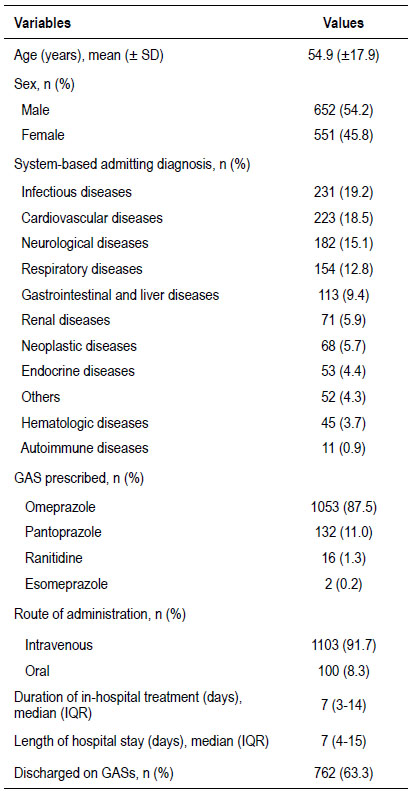
GASs, gastric-acid suppressant; IQR, interquartile range
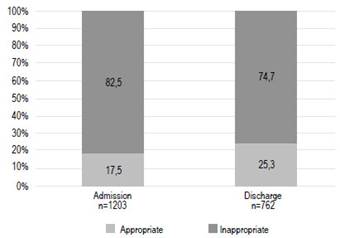
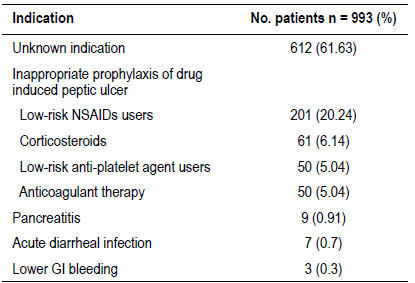
The prescriptions of GASs of two hundred ten (17.5%) patients were grouped into appropriate prescribing category in accordance with the approved indications (Figure 2). The most common indication was prophylaxis of peptic ulcers in patients aged > 65 years under treatment with NSAIDs/antiplatelets (38.57%), followed by treatment of peptic ulcers (35,24%) (Table 3). In contrast, 993 out of 1203 patients (82.5%) had inappropriate GASs prescribing. Most of these patients (61.63%) were given GASs for unknown reasons. Prophylaxis of peptic ulcers in low-risk NSAIDs users was the most frequent no evidence-based indication (20.24%) (Table 4). Seven hundred sixty-two patients were discharged on GASs. Among these patients, 74.7% (n=569) had no evidence-based reasons to continue this treatment at home (Figure 2).
Table 3 Evidence-based appropriate indication for gastric-acid suppressants.
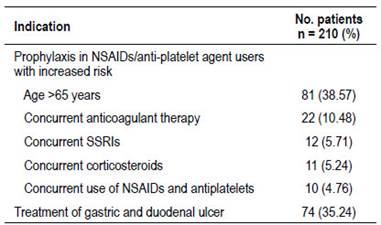
NSAIDs, nonsteroidal anti-inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors.
DISCUSSION
In this retrospective record review study, an overuse of GASs was found in most patients admitted to the internal medicine service (82.5%), with PPIs being the most frequent group of GASs prescribed. These results are consistent with those of other previous studies. For instance, a study carried out in a Spanish internal medicine service reported 73.03% of inappropriate prescribing of PPIs24. Another retrospective clinical record review study performed in a tertiary teaching hospital in Singapore evidenced that 81.2% of the patients aged ≥65 years admitted did have no an appropriate PPIs indication according to clinical guidelines25. In addition, Guptaet al.found that 73% of the patients who were started on GAS in an academic US hospital lacked accepted indications for their use26.
The current study showed that prophylaxis of drug-induced ulcers in low-risk patients was the main identifiable reason for the inappropriate prescribing of PPIs (36.46%). We found that many of these prescriptions were related to the use of NSAIDs/antiplatelet in lowrisk patients, anticoagulant therapy, and corticosteroid use alone. These findings are like those reported by Meliet al., who performed a one-day observational study and found that 40% of inappropriate PPI prescriptions were due to inappropriate prophylaxis of druginduced ulcers27. In a retrospective study carried out in a Dutch hospital, van den Bemtet al.reported that nearly half of the hospitalized patients who were newly prescribed NSAIDs received PPIs not in accordance with guidelines. Additionally, those authors found that overprescribing was found to be significantly associated with coxibs use and polypharmacy28.
It is noteworthy in this study that more than half of the patients with inappropriate GAS prescriptions had no documented reason for their use. We theorized that this fact could be related to the no-evidence belief that patients in hospital have a higher risk of developing peptic ulcer due to polypharmacy or stress ulcer outside a critical care setting.
We found that around 75% of the patients discharge on GASs did have no supported medical evidence to continue the acid suppressive therapy. This rate is similar to that reported by Phamet al., who documented that 79.1% of the patients that started PPIs continued this medication at discharge without having evidencebased reasons5. This can be explained by the fact that there is no established program in Venezuela for the deprescribing of GASs.
In Latin America, there are few studies about the overuse of GASs. Posadaet al.carried out a crosssectional descriptive observational study in a teaching hospital in Colombia and found that the prevalence of inappropriate prescribing of GASs was 59.5%, with prophylaxis of bleeding due to gastrointestinal ulcers in low-risk patients being the most frequent indication3. Similar findings were recorded by Bustamante-Robleset al.in two teaching hospitals in Lima, Peru, where the prevalence of inappropriate use of PPIs was 54,57%29.
The main strength of the current investigation is that, to the best of the authors’ knowledge, it was the study with the largest sample to assess the appropriateness of GASs prescribing in Latin America. There are several limitations in the present study that need to be considered. First, being a retrospective record review study, some information might not have been recorded. Indeed, this fact could be related to the high proportion of patients without a documented indication for the use of GASs. Second, in view of the retrospective design of our study, it is not possible to evaluate the decisionmaking process for the use of GASs. Third, we were unable to assess the duration of the treatment with GASs after the discharge because that information is not signaled in the medical records. This information is another variable to consider to assess the adequacy of treatment with GASs. Finally, the possible factors that could influence the inappropriate use of GASs were not evaluated in this study.
CONCLUSIONS
In summary, the present investigation illustrates that there is a high proportion of patients in a Venezuelan academic tertiary healthcare center that were prescribed GASs during their hospitalization not in accordance with the current clinical practice guidelines. This paper supports what prior studies in Latin America have reported about the high prevalence of inappropriate prescribing of GASs. Further research should be conducted to identify the possible causes of this overprescribing in order to develop regional strategies for the rational use of these drugs.