INTRODUCTION
Cystic lymphangiomas (CL) are an extremely rare sub type of slow-growing benign congenital malformations of the lymphatic system. Notably, to date, less than 200 cases have been reported in the literature 1. Pathophysiologically, this entity originates secondary to an obstruction of the lymphatic ducts, resulting in lymphangiectasia. Microscopically, CL are arranged as cystically dilated vascular channels lined by endothelial cells and filled with proteinaceous fluid 2. CL most frequently develop in the pediatric population. About 75% of all CL are found in the head or neck, 20% in the axillary regions and around 5% are found in the abdomen. RCL account for less than 1% of all lymphangiomas 4,5.
RCL are usually asymptomatic due to its slow-growing nature and often discovered incidentally. RCL may produce symptoms if they become large enough to compress on neighboring structures within the abdomen. The clinical presentation can range from vague abdominal pain, distention, fatigue and weight loss; to signs of acute abdomen secondary to RCL complication (hemorrhage, rupture, volvulus or infection) 5. Diagnostic techniques include ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI) and endoscopic ultrasound with cyst fluid fine-needle aspiration. However, the final diagnosis should always be confirmed by histology 6.
In this report, we document the case of a histologically confirmed-RCL in a 58-year-old cirrhotic patient with a history of a palpable right lower quadrant mass and abdominal pain. To our knowledge, this is the first case of a RCL in a cirrhotic patient reported in the literature. Written consent for publication was provided by the patient.
CASE DESCRIPTION
A 58-year-old Hispanic female has been followed up by our gastroenterology team at the outpatient clinic since 2018. She developed end-stage liver disease (ESLD) secondary to non-alcoholic steatohepatitis (NASH), which was complicated by hepatic encephalopathy and clinically significant portal hypertension. Her current medications include: carvedilol, rifaximin and lactulose. Her model for ESLD (MELD) score ranged between 11 to 13 since her first visit. She developed symptoms of recurrent mild dull right lower quadrant abdominal and pelvic pain that was associated with abdominal distension and constipation. These symptoms were ongoing during the next two years.
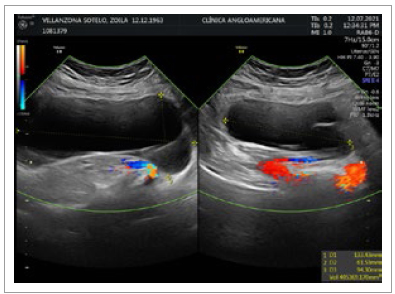
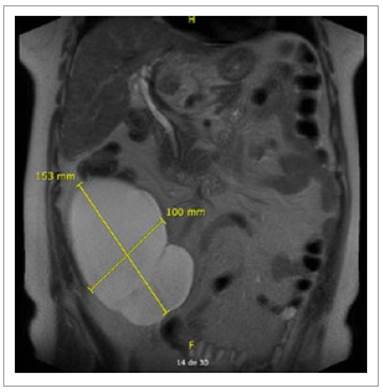
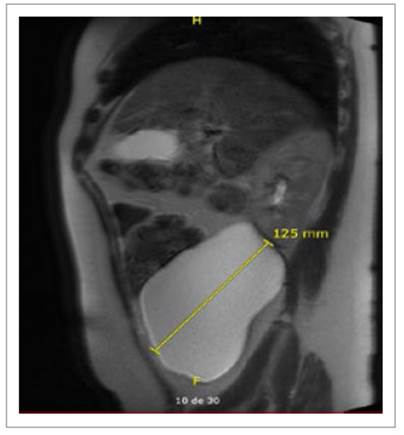
On June 2021, the patient was seen by her gynecologist for a routine checkup. Abdominal US showed a thin-walled, doppler negative tri-lobulated (140 x 94 x 64 mm.; 405 cc.) cyst on the right flank and lower right iliac region (Figure 1). At that time, invasive measures were dismissed due to her past medical history and minimal complaints. On July 2022, during follow-up visits with her gastroenterologist, the cyst was found to have increased in size (142 x 132 x .69 mm.; 660 cc.) and abdominal/pelvic symptoms worsened. An MRI scan described a large lobulated thinwalled complex cyst of 150 x 122 x 103 mm. without contrast enhancement located in the right flank as well as the retro cecal and retro colic spaces (Figures 2and 3) with no evidence of either small/large bowel, urinary tract or reproductive system involvement. The surgical team was then consulted and a decision was made to prepare the patient for an elective surgery.
The patient underwent an exploratory laparoscopy in an attempt to completely excise the retroperitoneal cyst. Upon laparoscopy, the liver showed a diffusely nodular and firm surface as well as multiple signs of collateral circulation. A large 150 x 150 x 120mm. cystic mass was then evidenced. The cyst compressed the right colon, small intestine as well the right kidney and ureter. The whole cyst mass was removed from the surrounding structures using a combination of sharp and blunt dissection. Histopathological examination (Figures 4 to 7) of the cystic wall revealed RCL and the patient was discharged on postoperative day 7 without further complications. Currently, the patient remains symptom-free at 7 months after surgery.
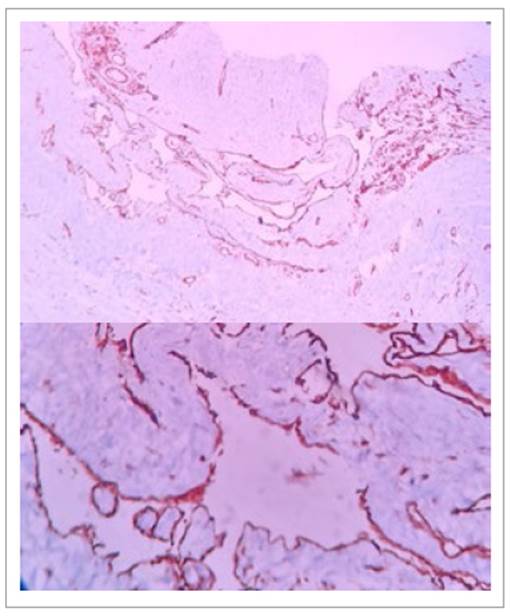
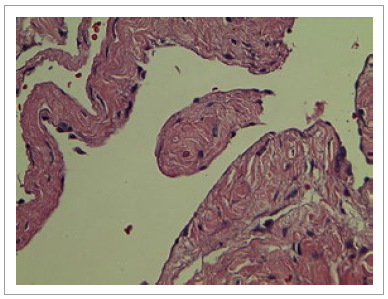
Figure 4 and 5 Histologic findings showing (10x and 40x slides respectively) a multi-cystic lesion formed by dilated cystic spaces with fibromuscular walls of variable width surrounded by simple flat epithelium without cytological atypia. Immunohistochemistry shows CD31 positive endothelial cells.
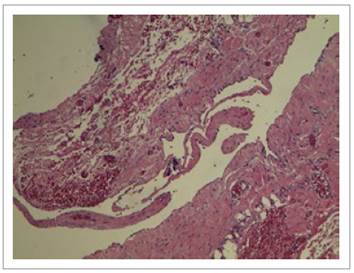
Figure 6 Histologic finding showing (10x) a hematoxylin and eosin-stained slide with a cystic space surrounded by simple flat epithelium compatible with endothelium. Fibromuscular wall of variable width.
DISCUSSION
Establishing an adequate protocol in order to diagnose and/or treat an uncommon disease is always problematic. In this report, our patient presented with a past medical history of a CHILD B, MELD 13, NASH-related liver cirrhosis and had been regularly monitored by our gastroenterology department. During her initial evaluation, a multiloculated cystic tumor was observed in the lower right hemiabdomen, which was initially believed to be an ovarian cyst. Over the course of the following 2 years, the patient reported experiencing dull abdominal pain that was predominantly localized to the lower right hemiabdomen; abdominal distension, constipation, and a palpable mass in the affected area. Due to the absence of any obvious malignant features and the initial clinical suspicion of a benign gynecological origin, outpatient monitoring was chosen as the initial approach. However, as the symptoms continued to worsen, a decision was made to reevaluate the case. MRI accomplished at that time showed a large retroperitoneal cystic mass. It is important to emphasize that the finding of a retroperitoneal cystic tumor, accounts for a wide variety of different diagnoses -e.g. mesothelioma, teratoma, liposarcoma, leiomyosarcoma, lymphangioma, adenoma, duplication cysts, hydatid cysts (very common in Peru and should always be part of the differential diagnosis), abscesses, among others. This is especially challenging in the setting of a cirrhotic patient.
Notably, a pre-operative diagnosis of RCL is extremely difficult to obtain and a definite diagnosis is habitually made by histological examination of the specimen following surgery. Thus, an optimal pre-operative radiological evaluation is essential to be able to better specify the diagnosis. US, CT scan and MRI have demonstrated to be complementary to each other in the diagnosis of RCL. US can show the internal structure of lymphangiomas, its septations and particularly, the cystic nature of the lesion. CT scan can reveal uni- or multi-locular cysts with septae, and most importantly, can assess the relationship of lymphangiomas to neighboring organs 7. Similarly, MRI plays a crucial function in the evaluation of retroperitoneal cystic masses as it can provide information on the internal composition of these lesions, which is determinant for diagnosis, and for instance, future therapy. Furthermore, in the case of retroperitoneal cystic tumors that will undergo surgical treatment, MRI is fundamental for identifying the location and extent of the lesions and consequently, allowing for a more accurate treatment planning 8. Lymphangiomas typically appear on MRI as wellcircumscribed multicystic masses that extend between adjacent organs with thin, smooth enhancing walls and septations.
They can span multiple compartments and cross fascial planes, distinguishing them from other retroperitoneal cystic lesions. On T1-and T2-weighted images, lymphangiomas appear homogeneously hypointense and hyperintense, respectively, without internal enhancement or nodularity 8. In our patient, MRI showed a 150 x 122 x 103 mm. retroperitoneal complex cystic mass with no internal enhancement.
As a "general rule" in retroperitoneal (cystic and noncystic) masses, tumor size is a determining factor for the development of symptoms. For instance, tumors smaller than 15-20 cm. are often found incidentally and are associated with only mild complaints. On the other hand, tumors larger than the afore-mentioned size, tend to manifest with at least one of the following problems: a) an increase in abdominal diameter and/or evidence of a palpable mass (the most common sign) 9, b) weight loss associated with the tumor may be compensated by the increase in weight generated by the tumor growth 10, c) signs of extrinsic tumor compression such as phlebitis, edema in the lower extremities, compression of the nerves, and early digestive or urinary symptoms 10, d) abdominal and lumbar pain are often present 11 and e) patients frequently present with early satiety, nausea, and vomiting associated with tumor compression 10.
Therefore, having the case of a patient like ours with a large retroperitoneal cystic tumor that was causing her a lot of discomfort (abdominal pain and distension); the decision was made to completely excise the mass using a laparoscopic approach. The patient tolerated the procedure very well without major complications. In fact, a complete radical surgical resection is the recommended treatment for RCL 12 as: a) will drastically reduce the chances of complications (e.g. superinfection, progressive growth, rupture or bleeding) and b) patients usually have good post-surgical outcomes 13. Moreover, when possible, a complete resection must be ensured as incomplete resections are associated with an increased risk of recurrence (50% for incomplete vs 7% for complete resections) 14,15. Other more "conservative" methods have also been described in the literature -e.g. cyst enterostomy, aspiration and peritoneal marsupialization; however, nowadays, they have become obsolete due to high recurrence rate 16,17.
As mentioned before, histological confirmation is mandatory in order to obtain the final diagnosis of RCL. Briefly, Wegener divided all lymphangiomas into 3 main categories: a) lymphangioma simplex (capillary lymphangioma) with small, thin-walled lymphatic channels; b) cavernous lymphangioma with larger thinwalled channels (may undergo malignant transformation) and c) cystic lymphangioma (always benign) composed of large cystic spaces lined with flat endothelium. Retroperitoneal lymphangiomas are usually of cavernous or cystic types 18. Immunohistochemically, the markers of lymphatic vessel endothelial receptor-1, vascular endothelial growth factor-3, prox-1, and monoclonal antibody D2-40 are used to diagnose lymphangiomas by identifying specific endothelial cells of lymphatic tissues 5. Likewise, RCL endothelial cells may also express factor VIII-related antigen, CD31 and CD34 19. Accordingly, histological examination of the cystic mass obtained from our patient showed a multi-cystic lesion formed by dilated cystic spaces with fibromuscular walls of variable width surrounded by simple flat epithelium without cytological atypia; immunohistochemistry confirmed CD31 positive endothelial cells. Consequently, the diagnosis of RCL was corroborated.
The etiology of lymphangiomas still remains unclear. The most accepted theory proposes that they originate from the early sequestration of lymphatic vessels that fail to establish connections with normal draining lymphatics at about14-20 weeks of intrauterine life 20. Hence, lymphangiomas are considered congenital tumors. Others hypothesis regarding their origin include obstruction of lymph channels secondary to fibrosis, inflammation, trauma, node degeneration; or failure of endothelial secretory function 21.
Lymphangiomas are usually present at birth and, in fact, in approximately 90% of the cases, they are diagnosed prior to 2 years of age 3. Clinically, they appear as a large, poorly delimited, translucent, soft mass covered by normal skin, most commonly located in the cervicofacial region (75%), axilla (20%), or lateral chest wall. This tumor can be detected prenatally in the first trimester of pregnancy and, in approximately 50% of cases, is associated with chromosomal abnormalities, such as Down syndrome, Turner syndrome, and Noonan syndrome 22. As stated before, adult presentation is atypical. Even though, lymphangiomas can theoretically develop at any intraabdominal location; the retroperitoneum accounts for less than 1% of all the cases. At the time of this report, less than 200 cases of RCL have been reported in the literature.
Finally, it was difficult for our group to attribute all of the symptoms that our patient developed to RCL alone; mainly because of the co-existence of liver cirrhosis, as this disease itself could easily add symptomatology to the clinical presentation.
In conclusion, RCL is an uncommon disease in adults; even more if they occur in a cirrhotic patient. In fact, at least to our knowledge, this is the first case described in the literature. Optimal radiological evaluation provides vital pre-operative diagnostic information for successful management; however, histological confirmation is always needed in order to obtain the final diagnosis. The recommended treatment is complete surgical excision and prognosis is excellent.