INTRODUCTION
Gastric cancer (GC) is a multifactorial disease with important genetic and environmental factors 1. Around the world, it is the fifth most common cancer in incidence and the fourth cause of death secondary to cancer, being its presentation more common in men 2). It is more common in regions of Eastern Europe, East Asia, and Latin America, mainly due to the diet characteristics of these populations and a high rate of Helicobacter pylori infection 3). According to data from the Global Cancer Statistics (GLOBOCAN), in 2020, Colombia had 8,214 new cases of GC (7.3% of total cases) with a 5-year prevalence of 22.82 cases per 100,000 inhabitants; being in Colombia, the leading cause of mortality secondary to cancer (6,451 cases, 11.7% of the total deaths) 2). Recently, a clinical practice guideline for primary and secondary prevention and early diagnosis of gastric cancer was published in Colombia. However, there are no recommendations on screening for this neoplasm in the general population in asymptomatic patients. In addition, in the cases of patients with first-degree relatives with gastric cancer, they mention that there is no evidence either for or against doing an active search through upper digestive endoscopy, so cases must be individualized 4).
Most GC are adenocarcinomas, which can be divided into two groups depending on whether the tumor is in the cardia region 5). These entities differ in risk factors, carcinogenesis, and epidemiological patterns 6). During the last decades, the histological classification of Lauren, with the classification of intestinal, diffuse, and mixed type GC, has been the most straightforward and most robust classification for the clinical, endoscopic, and therapeutic approach of patients with GC 7).
The presentation of GC cancer in those younger than 30 years is uncommon. However, the incidence increases and histopathological characteristics of GC at the time of diagnosis in a Latin American population.
MATERIALS AND METHODS
Patients
A retrospective and descriptive cross-sectional study was carried out using the database of the Gastroenterology Service of the Clínica Foscal and Clínica Foscal Internacional in Bucaramanga, Colombia. A total of 4,041 esophagogastroduodenoscopies (EGD) were performed in the medical centers between January 2016 and December 2019 under any medical indication.
The inclusion criterion for the study was patients over 18 years of age with no previous history of GC in whom EGD had been performed as indicated by the Emergency Department or Hospitalization Medical Team. The exclusion criteria were records of EGD performed on an outpatient basis or indicated in an outpatient clinic, a history of human immunodeficiency virus (HIV) infection, a history of solid or hematological organ transplantation, and treatment with immunosuppressive drugs.
The information was collected in a format specifically designed for this study. Among the variables that are taken into account are sociodemographic information, the presumptive diagnosis considered as an indication for the endoscopic procedure (of the International Classification of Diseases, tenth edition (ICD-10)), the clinical characteristics (symptoms reported by patients during EGD), the macroscopic morphological findings represented by the anatomical location of the lesion and the endoscopic pattern observed (Paris classification for early neoplastic lesions, and Borrmann designation for rapidly and steadily after this decade to reach the highest advanced lesions) 15,16), and the microscopic morphology rates in the older age groups 8). The definition of early- onset gastric cancer (EOGC) is controversial, and there is no consensus on the cut-off age. Our study had a cut-off of 40 years because this was used by Gomez et al. 9), who studied a population like ours, in addition to other reports worldwide 10,11).
The incidence and clinicopathological characteristics of EOGC are not relatively well defined. According to the Surveillance, Epidemiology, and End Results (SEER) database, a continuous increase in the rate of EOGC has been observed since 1995, now being over 30% of GC cases in the United States 12). The incidence varies depending on the population studied and is between 2% to 15% 13). According to some studies, it has been seen that EOGC is associated with a more remarkable presentation in women and with the presence of diffuse histology; In addition, it is unclear if it has a more aggressive behavior than the usual GC presentation 14).
For this reason, the objective of this study was to evaluate the association between age and clinical, endoscopic, according to Lauren's classification 17. A database was created with the variables to be studied.
Statistical analysis
Bivariate comparison of the frequency of the clinical, endoscopic, and histopathological characteristics of GC was performed according to the age at diagnosis before or after 40 years, in the form of graphs and tables, using frequency measures for the qualitative variables and measures of central tendency or quartiles for the quantitative variables. For all comparisons, an α significance level of 0.05 was applied. The χ2 test or Fischer's exact test was obtained for comparisons between groups. For all analyses, the statistical package Stata 14 (created by StataCorp LLC, College Station, Texas, USA) was used.
RESULTS
In total, 259 cases of de novo GC were included in the study. The median age was 62 years at diagnosis, with an interquartile range (IQR) between 49 and 73 years. 36 patients (13.9%) were 40 years old or younger, compared with 223 patients older than 40 years. GC was found more frequently in men (n=149, 57.53%) than in women (n=110, 42.47%). However, it was evidenced with a statistical difference that in patients under 40 years of age (36 cases), the prevalence of GC diagnosis was lower in men ([PR, 95% CI] = 0.53 [0.29-0.97]). Family history of GC or any other neoplasm was not associated with our population's higher prevalence of GC.
Among the most common symptoms reported were dyspepsia (n=157, 60.6%), weight loss (n=113, 43.62%), and abdominal pain (n=108, 41.69%). Other symptoms were gastrointestinal bleeding, anemia syndrome, vomiting, dysphagia, ascites, and abdominal mass (Table 1). When performing the statistical analysis of symptoms in the population under 40 years of age, it was shown that the presence of vomiting ([PR, 95% CI] = 2.24 [1.18- 4.26]) and ascites are associated with a higher risk of having GC ([PR, 95% CI] = 3.67 [1.91- 7.04]).
Table 1 Clinical characteristics of gastric cancer patients.
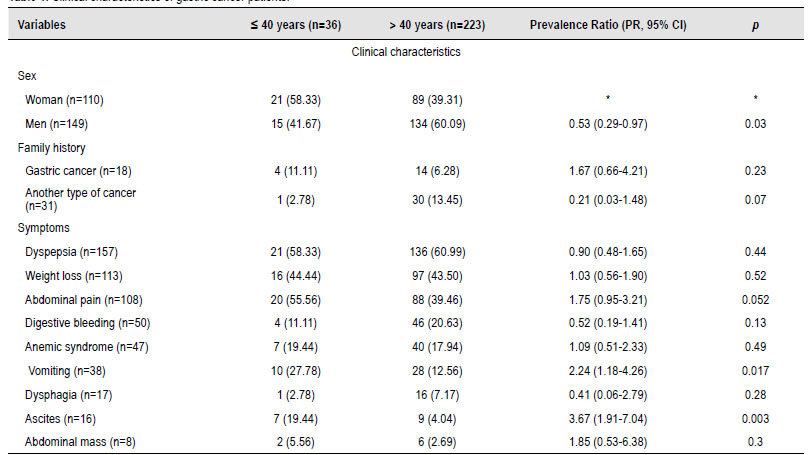
*In some variables, there was no Prevalence Ratio (PR) calculation and statistical p-value because they were used as a compared variable or there was no comparison group.
During the endoscopic procedure, it was evident that the neoplasms were located in most of the patients in the body (n=167, 64.47%) and antrum (n=132, 50.96%). When comparing by age group, it was statistically significant that patients under 40 years of age will have a location more commonly in the gastric body ([PR, 95% CI]=2.28 [1.04-5.01]). In the endoscopic classification of the lesion, most of the lesions were advanced (n=253, 97.68%), with the Borrmann III classification being more prevalent (n=124/253, 49.01%). It was evident that in patients under 40 years of age with GC, it was less likely to be of Borrmann II classification ([PR, 95% CI] = 0.23 [0.06-0.94]); but on the contrary, patients younger than 40 years were more likely to have a Borrmann IV endoscopic lesion ([PR, 95% CI]=1.89 [1.03-3.46]). Histological analysis showed that most patients presented intestinal-type characteristics (n=139, 53.66%); however, when comparing patients with early-onset GC, it was found that this population was more likely to have diffuse-type histology ([PR, 95% CI]=7.16 [2.85-17.99]) or mixed ([PR, 95% CI]=7.32 [2.33-22.94]) than in patients older than 40 years (Table 2).
Table 2 Endoscopic and histological characteristics of gastric cancer patients.
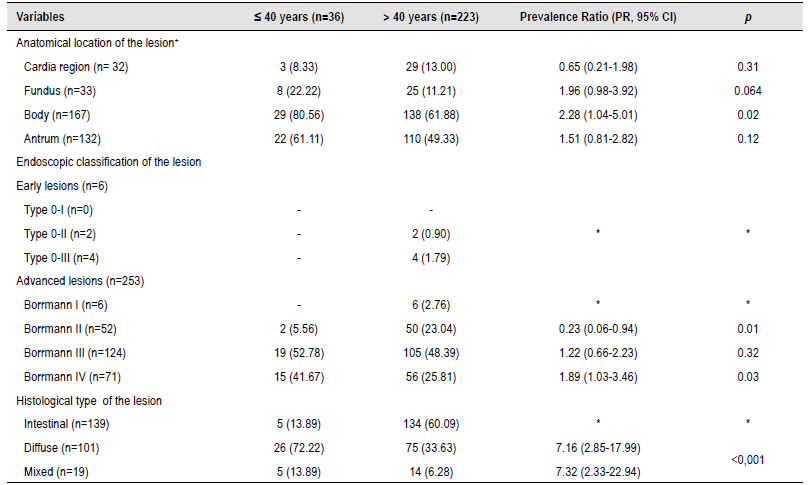
*In some variables, there was no Prevalence Ratio (PR) calculation and statistical p-value because they were used as a compared variable or there was no comparison group.
+Numbers do not add up in the anatomical location of the lesion since patients might be included in more than one cell.
DISCUSSION
GC is frequently diagnosed in the middle age and elderly, with a peak incidence between 50 and 70 years 18). Our study found that 13.9% of GC cases were developed in a population aged 40 years or younger. Gomez et al.(9 set a study in a population like ours and reported a percentage of 8.8% of EOGC. It has been suggested that EOGC has different clinical characteristics and tumor behavior than GC in older adults.
In our study, it is evident that the percentage of cases of EOGC is higher in the female population (58.3%), which is consistent with previous studies, such as that of Hsieh et al.(19), where they analyzed a sample of 1815 cases of GC with 115 cases of EOGC, reporting that 60% (n=69) of these cases occurred in the female population. Similarly, a prospective study with a European population found that it was statistically more common to find cases of EOGC in women (57%, p=0.004) 20).
GC is considered to develop due to a combination of genetic and environmental factors, with hereditary involvement in less than 3% of GC cases, being most genetic alterations acquired 21). Setia et al. 22 recently conducted a cohort study studying genomic alterations in patients with EOGC. The study showed genetic changes in 69 genes, with the mutation in TP53 being the main one (63%), followed by changes in the CDH1 gene (together with TP53 in 12.3% of cases and without TP53 in 9.9%). In our study, no association was found between the appearance of EOGC and a family history of GC or another neoplasm. Bergquist et al. 12 survey is one of the most extensive registry studies, with cross-sectional data on risk factors and detailed genomic analysis for GC. They reported a lack of association of GC risk factors in general (smoking, diabetes, obesity, alcoholism) with the presentation of EOGC. The effect of the interaction of H. pylori with the gastric mucosa is one of the most critical factors thought to be involved in the development of GC in the young population 1). Masuda et al. (23 found that the presence of EOGC correlates with H. pylori but not with family or genetic background. There are contradictory data, such as the study by Marcos-Pinto et al. 24), which showed that first-degree relatives of patients with EOGC have different phenotypic and molecular profiles. Their research reported that H. pylori (vacA s1 and vacA m1 strains) was present in 82% of cases (versus 62% in controls, p= 0.004).
The most frequent symptoms found in our study population were dyspepsia, weight loss, and abdominal pain. Alarm symptoms, accompanied by dyspeptic symptoms, have a diagnostic role by indicating the possible presence of a neoplasm. Further, when they are present at the time of diagnosis in patients with GC, they usually suggest the location and aggressiveness of cancer, indicating, in most cases, poor prognosis 25). In our study, vomiting and ascites were statistically significantly more frequent in the population under 40 years of age. Braga- Neto et al.(26) conducted a six-year prospective cohort study in patients with EOGC (n=41) and older patients with GC (n=166). They reported that the most prevalent symptoms in young patients were abdominal pain (90.2%), weight loss (75.6%), and vomiting (65.9%). As in our study, ascites finding was more common in the young population group; however, it was not statistically significant.
In the patients included in our study, it was evidenced that the anatomical location of the tumor predominated in the body and the antrum of the stomach for all patients. In young adults, a significantly higher proportion of lesions were found in the gastric body. In the meta-analysis carried out by Kong et al. 27) regarding tumor location in patients with GC, six studies with 7,131 patients were included. The proportion of young patients with a tumor located in the middle third of the stomach (29.22%, 371/1,270 versus 22.95%, 1,345/5,861; pooled OR 1.66; 95% CI: 1.43-1.92; p<0.00001) was reported to be significantly higher than in older patients. Most patients, regardless of age, endoscopically presented advanced-stage lesions, predominantly Borrmann classification type III (n=124) and type IV (n=71). From our analysis, according to the age at presentation of GC, it can be considered that patients were less likely to find Borrmann II type lesions and, conversely, more likely to present Borrmann IV type lesions. In the study by Isobe et al. 28), Borrmann IV lesions were more frequent in patients younger than 40 years (n=169) (23.7 versus 9.5%, p <0.0001), while Borrmann lesions 0, I, and II were more common in patients older than 40 years (n=3649) (36.7 versus 46.2%, p=0.016; 0 versus 2.4%, p=0.042; 6.5 versus 11.8%, p=0.035, respectively). Similarly, in the meta-analysis by Kong et al. 27), Borrmann IV classification was more common in young patients (11.06%, 206/1,863 versus 7.96%, 470/5,908; pooled OR 1.66; 95% CI: 1.24-2.23; p<0.0007).
The histological analysis of this study found that according to Lauren's classification, patients with EOGC have a higher diffuse or mixed-type histology prevalence. This finding is consistent with previous studies, such as the one by Braga-Neto et al. 26), in which diffuse infiltrative histology was more common in young patients than in older patients (70.70% versus 33.70%, respectively; p<0.001). Similarly, Hsieh et al. 19) also showed in their study that the presence of diffuse histology was more common in patients under 40 years of age (55.6% versus 27.7%; p < 0.0001).
This study is of great importance as it shows that the percentage of de novo EOGC is remarkable. However, the demographic information on this disease is unknown in our country. This article is the first report on EOGC in the population of northeastern Colombia and the second in Colombia 9). This study had limitations in its conduct and analysis. First, due to its cross-sectional nature, it is impossible to give conclusions with more statistical weight regarding the association of factors. This is why new longitudinal studies with larger sample sizes, levels of evidence, and degrees of recommendation are justified. Similarly, our study was conducted only with hospitalized patients with symptoms or signs that warranted EGD, so we did not include asymptomatic patients. In addition, in our research, the analysis of endoscopic results was predominant, so no tumor extension or staging variables were obtained, which we consider being of great interest in patients with EOGC in subsequent studies.
We can conclude that this study made an approximation to the characteristics of the EOGC in Latin America. We report that in our population, EOGC is more common in women, with a preferred anatomical location in the body of the stomach, with Borrmann IV classification and diffuse-type histology being more likely. Expanding the research horizon also toward the young population and implementing strategies to prevent, control, and detect this disease early is essential in the future.