INTRODUCTION
Plexiform fibromyxoma (PF) is a rare mesenchymal neoplasm most commonly arising in the gastric antrum, but it may also occur in the pylorus, duodenum, jejunum, colon, and biliary tract. PF affects adults of either sex occurring over an age range of 40 to 50 years, although it may also occur in children 1,2). Tumor size ranges from 0.3 to 17 cm 3). PF presents as a solid, solid/cystic, or cystic mass on computed tomography (CT), magnetic resonance imaging (MRI) and Endoscopic ultrasound (EUS). Many of these lesions are misdiagnosed as gastrointestinal stromal tumor (GIST) because they appear as a lobulated submucosal mass, most commonly ulcerated, with a risk of gastrointestinal bleeding and consequent anemia. Most GISTs are positive for CD117, DOG1, and CD34 4). Symptoms include abdominal pain and nausea, occasionally but PFs can also be found incidentally.
CASE REPORT
A 56-year-old white female patient presented 4 years ago to the Department of Digestive Endoscopy of Hospital Santa Casa de Caridade, in Bagé, southern Brazil, with a 30-day history of nausea preceded by relevant past medical history. Gastroscopy (LASEREO L590ZW, Fujifilm Co) at that time revealed a subepithelial lesion measuring approximately 20 mm in diameter, located in the posterior wall of the antrum, with leakage of serous fluid after biopsy (shown in Figure 1).

Figure 1 (A) Subepithelial lesion in the posterior wall of the antrum. (B and C) Leakage of serous fluid after biopsy
Histopathology showed only a gastric inflammatory process, possibly because only superficial biopsies were performed and with the presence of Helicobacter pylori.
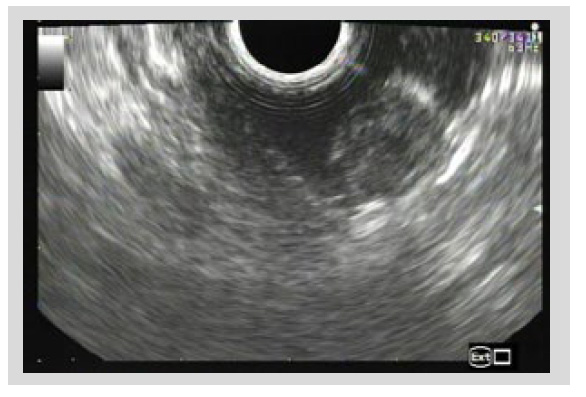
Endoscopic ultrasound (EUS) revealed a 2 cm heterogeneous hypoechoic mass with cystic components arising from the second layer of the gastric wall with no disruption of the proper muscle layer, suggested of ectopic pancreas (shown in Figure 2).
Follow-up gastroscopies performed 24, 36, and 48 months later showed a subepithelial lesion in the posterior wall of the antrum, with slight scar retraction in the center, measuring approximately 12 mm in diameter (smaller than the mass detected at index endoscopy), without leakage of serous fluid (shown in Figure 3).

Figure 3 (A and B) Follow-up gastroscopy showing the same subepithelial lesion, with slight scar retraction in the center, smaller than the mass detected at index endoscopy, without leakage of serous fluid after biopsies.
Surveillance biopsy bite-on-bite with large capacity forceps was performed at each follow-up gastroscopy. Histopathology was inconclusive, except for the last follow-up, showing bland spindle cell proliferation, with a vaguely plexiform/multinodular pattern, in a fibromyxoid stroma with an arborizing capillary network and no mitoses identified (shown in Figure 4). Cellularity was generally low. Immunohistochemically, the tumor cells were positive for smooth muscle actin (SMA) and negative for DOG1, CD117, CD34, S100, desmin, epithelial membrane antigen (EMA), CD10, calponin, and beta-catenin (shown in Figure 4). These findings were suggestive of PF.

Figure 4 Histopathology and immunohistochemistry. (A, B and C) Presence of bland spindle cell proliferation, with a vaguely plexiform/multinodular pattern, in a fibromyxoid stroma showing an arborizing capillary network and absence of mitosis (hematoxylin and eosin). (D and E) The cells are positive for SMA (400x). (F) The cells are negative for CD34 (400x).
Because no cases of malignant transformation have previously been reported and given the features of this case, with a reduction in lesion size and no ulceration, and after discussing with the patient, a decision was made to maintain follow-up.
This case report was written in accordance with the World Medical Association Declaration of Helsinki. Informed consent was obtained from the patient.
DISCUSSION
Takahashi et al. 5 first described it in 2007 as a plexiform angiomyxoid myofibroblastic tumor. In 2009, Miettinen et al. 6 described 12 cases of the same tumor in the antrum and named it as PF, the terminology adopted in the 4th edition of the WHO Classification of Tumors of the Digestive System in 2010 1). The most common clinical features are hematemesis and anemia, and the patient may complain of abdominal pain and nausea or even be asymptomatic. Our patient reported only nausea, showing improvement after proton pump inhibitor. Bleeding is usually associated with the presence of ulceration, with endoscopic findings suggestive of GIST, as irregular border and lobulated subepithelial mass, and occurs in about 50% of cases 3). Histologically, PF is characterized by a plexiform growth pattern, ovoid to spindle cell proliferation, and a myxoid stroma rich in small blood vessels, without substantial cytological atypia and with low mitotic activity. Intravascular involvement has been described, suggesting a possible intravascular spread of the tumor within the gastric wall to the subserosa 6).
The diagnosis is made by immunohistochemistry, with the tumor cells being positive for SMA and vimentin and negative for CD117, DOG1, CD34, S100, beta-catenin, STAT-6, and ALK. There was immunoreactivity for SMA in the case reported here, with the tumor cells being negative for all other immunostains. GIST is the most common mesenchymal tumor of the stomach and the main differential diagnosis of PF. GISTs are more aggressive than PFs and positive for CD117, CD34, and DOG1 stains, whereas schwannomas are positive for S100 and SOX10. Therefore, negative staining for CD117 and DOG1 excludes GIST, whereas negative staining for S100 excludes neural tumors. Vascular lesions are positive for CD34 and CD31 4,7). EUS often shows a hypoechoic mass arising from the muscle layer. Fine-needle aspiration can either support or mislead the diagnosis. A meta-analysis evaluating EUS tissue acquisition for upper GI SELs presented a diagnostic rate of only 59.9% 8). FNA was not employed due to the appearance of ectopic pancreas. CT and MRI are important noninvasive imaging modalities for diagnosis, especially of exogastric tumors, and treatment planning 9). ESGE consider EUS as the best tool to characterize SELs, and it recommends EUS-guided fine-needle biopsy (EUS-FNB) or mucosal incision-assisted biopsy (MIAB) for tissue diagnosis of SELs ≥20 mm and MIAB as first choice to SELs < 20 mm, as bite-on-bite, jumbo, snare or submucosal tunneling 10).
In the series of 10 cases reported by Hu et al. 9), average tumor size was 3.2 cm, 8/10 (80%) gastric lesions were located in the antrum, 7/10 (70%) patients reported abdominal pain or distension, and 1/10 (10%) patient was asymptomatic. All cases were strongly positive for vimentin and SMA 9). Until the review published in 2021 by Arslan et al. 11), 130 cases of PF had been reported in the literature, with only 19 cases being described between 2007 and 2010. A possible explanation for the increased diagnosis of PF may be the increased awareness of this pathology. Yang et al. 12 reported a subepithelial lesion located in the posterior wall of the upper gastric body, and EUS identified a heterogeneous hypoechoic mass arising from the muscularis propria layer, measuring 5.6 x 3.5 cm. Examination of the specimen showed a cystic portion containing mucinous fluid and a solid portion exhibiting a mucoid, gelatinous mass with extramural hemorrhage 12). In the case reported here, the lesion was subepithelial with leakage of serous fluid after biopsy, and EUS identified a heterogeneous hypoechoic mass with cystic components arising from the second layer of the gastric wall. Patient returned to follow-up only 24 months after index endoscopy. It was performed with biopsy bite on bite with large capacity forceps. ESGE suggests surveillance of asymptomatic gastric SELs without definitive diagnosis, with EGD at 3-6 months, and then at 1-2-year intervals for lesions 10-20 mm in size. The choice of treatment and follow-up depends on the SEL subtype, the layer of origin, location and local expertise 10).
Distal or partial gastrectomy is in cases of PF located in the antrum, with endoscopic submucosal dissection as an interesting less invasive alternative (9).
An incidental 2 cm gastric SEL, shown to be a plexiform fibromyxoma in gastric biopsies, with a benign behavior over the course of 4 years including a dimensional down size to 12 mm.