Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Anales de la Facultad de Medicina
Print version ISSN 1025-5583
An. Fac. med. vol.80 no.3 Lima July/Sep. 2019
http://dx.doi.org/10.15381/anales.803.15994
ARTÍCULO ORIGINAL
Non-clinical performance and acceptability of a small portable respiratory stimulator device for basic neonatal resuscitation, tested by health personnel
Desempeño no clínico y aceptabilidad de un estimulador respiratorio portaìtil para reanimación neonatal baìsica, evaluado en personal de salud
Carlos A. Delgado1,2,3,a, Víctor M. Sánchez1,2,3,b, Pablo M. Velásquez1,2,3,4,c, Roberto L. Shimabuku1,2,d, Luis Huicho2,5,e
1 Grupo de Investigación en Neonatología, Facultad de Medicina, Universidad Nacional Mayor de San Marcos. Lima, Perú.
2 Centro de Investigación en Salud Materna e Infantil, Centro de Investigación Para el Desarrollo Integral y Sostenible, Universidad Peruana Cayetano Heredia. Lima, Perú.
3 Instituto Nacional de Salud del Niño, Ministerio de Salud. Lima, Perú.
4 Instituto Nacional Materno Perinatal, Ministerio de Salud. Lima, Perú.
5 Facultad de Medicina, Universidad Peruana Cayetano Heredia. Lima, Perú.
a Médico neonatólogo, ORCID: https://orcid.org/0000-0002-6073-8109.
b Médico neonatólogo, ORCID: https://orcid.org/0000-0002-0965-310X.
c Médico neonatólogo, ORCID: https://orcid.org/0000-0003-1873-5390.
d Médico neonatólogo, ORCID: https://orcid.org/0000-0001-6156-6786.
e Médico pediatra, ORCID: https://orcid.org/0000-0002-5272-5885.
RESUMEN
Introducción: La reanimación neonatal demanda dispositivos para apoyo respiratorio que no siempre se encuentran en áreas rurales. Se requieren dispositivos innovadores y el prototipado rápido permite generarlos usando diseños e impresoras tridimensionales (3D). Objetivo: Evaluar el desempeño no clínico y la aceptabilidad por el personal de salud de un dispositivo respiratorio neonatal producido mediante prototipado rápido. Métodos: Estudio observacional, descriptivo, de prueba de concepto desarrollado en dos etapas. Etapa 1: Fabricación del dispositivo con prototipado rápido en impresoras y escáneres tridimensionales (3D). Etapa 2: Demostración del dispositivo durante programas de capacitación en reanimación neonatal para personal de salud en tres regiones del Perú (Tarapoto, Huánuco y Ayacucho). En ambas etapas se evaluó el desempeño del dispositivo conectado a un analizador de flujo de gases. Se administró una encuesta a los trabajadores de salud de Tarapoto y Ayacucho para conocer su aceptabilidad. Resultados: El prototipo desarrollado tiene forma de T con dos fuelles laterales que al presionarse con una sola mano, proyectan aire por el centro hacia un adaptador facial. El uso del prototipo en laboratorio generó un flujo de aire promedio de 4,8 Lt/min (DE ± 1,7) y una presión promedio de 5,9 cm H2O (DE ± 1,4). Este dispositivo fue considerado como "de uso muy simple" en una encuesta de aceptabilidad donde participaron 39 enfermeras y 11 médicos en zonas alejadas de la capital del Perú. Conclusiones: El prototipo evaluado fue aceptado por el personal y tuvo un desempeño capaz de generar un estímulo de la respiración espontánea al nacer.
Palabras clave: Asfixia Neonatal; Reanimación Cardiopulmonar; Dispositivo Médico (fuente: DeCS BIREME).
ABSTRACT
Introduction: Neonatal resuscitation demands equipment for respiratory support not always available in rural areas. Innovative devices are required, and rapid prototyping allows to generate them using three-dimensional (3D) designs and printers. Objective: To evaluate the non-clinical performance and the acceptability by health personnel of a neonatal respiratory device produced by rapid prototyping. Methods: Observational study, descriptive, of proof of concept developed in two steps. Step 1: Manufacture of the device with rapid prototyping in three-dimensional (3D) scanners and printers. Step 2: Demonstration of the invention during training programs in neonatal resuscitation for health personnel in three regions of Peru (Tarapoto, Huánuco and Ayacucho). In both steps, we evaluated the performance of the device connected to a gas flow analyser. A survey was administered to the health workers of Tarapoto and Ayacucho to know their acceptability. Results: The developed prototype is T-shaped with two side bellows that, when pressed with one hand, project air through the centre towards a facial adapter. The use of the prototype in the laboratory generated an average air flow of 4.8 Lt /min (SD ± 1.7) and an average pressure of 5.9 cm H2O (SD ± 1.4). This device was considered to be "very simple to use" in an acceptability survey involving 39 nurses and 11 doctors in remote areas of the capital of Peru. Conclusions: The evaluated prototype is acceptable by the staff and has a performance capable of generating spontaneous breathing at birth.
Keywords: Asphyxia Neonatorum; Cardiopulmonary Resuscitation; Medical Device (source: MeSH NLM).
INTRODUCTION
The World Health Organization (WHO), UNICEF and various international organisations promote effective neonatal resuscitation to prevent neonatal deaths and long-term disability (1,2). Other initiatives to help children breathe include the use of algorithms adapted for advanced resuscitation (3) or the teaching of basic neonatal resuscitation techniques in remote areas with limited resources (4). However, the coverage of these interventions is insufficient, especially in remote rural areas of low- and middle-income countries (LMICs) (5,6).
Basic neonatal resuscitation includes providing heat to prevent hypothermia, an adequate position to clear the airways, cleaning the upper respiratory tract if necessary, drying skin to avoid heat loss, and an additional tactile stimulation, to trigger the respiratory effort. If heart rate is less than 100 per minute or there is no adequate respiratory effort, positive pressure ventilation (PPV) must be started, ideally before the first 60 seconds of life (7,8). Advanced resuscitation includes endotracheal intubation, cardiac massage, and drugs administration, although the last two measures are exceptional, and for barely one out of every 1,000 births (8).
When PPV is required, WHO recommends using a self-inflating bag attached to a face mask (1). However, these selfinflatable bags show wide variability in their performance, require trained personnel, and are rarely available in remote and rural areas, where most births occur outside of health facilities (9,10). Thus the development and assessment of innovative, economical and practical alternatives for respiratory support at the time of birth is largely overdue.
Sparse evidence compares the performance of neonatal respiratory devices because the conventional design of the self-inflating bag has not changed much in recent decades (11). However, we found a study performed in Seattle (USA) by PATH, a nongovernmental organisation (12). Those researchers presented the comparison of performance and acceptability of two different self-inflatable bags. They compa-red the conventional horizontal and a vertical prototype of the neonatal bag. Both devices were able to deliver the minimum tidal volumes required for newborns. Two user groups participated in this evaluation: (1) frequent and trained users, and (2) infrequent users who had received training based on competitions but never used a bag-mask with a baby. The trained users include respiratory therapists, neonatologists and neonatal intensive care nurses who worked at Seattle Children’s Hospital. The latter group included students of respiratory therapy and obstetrics from local universities. Researchers found that vertical device provided a significant reduction in the percentage of inadequate ventilation, even in rare users. Subjective acceptance and disassembly/reassembly tests supported the vertical design of the device, although it was proposed to conduct clinical studies with infrequent users in low-income facilities (12).
Recently published guidelines recommend the use of ambient air, and supplemental oxygen is no longer the first choice as part of the resuscitation manoeuvres (13), which opens the door for a simplified yet effective neonatal care in remote areas. Several national and international studies have demonstrated the need to control the excessive use of oxygen in neonatal resuscitation (14-17). There is even evidence that the excessive use of oxygen can generate a physiological paradox that precipitates a significant clinical deterioration (18). The most recent clinical guidelines of the American Association of Cardiology indicate that it is reasonable to begin neonatal resuscitation without supplemental oxygen in full-term infants (8).
All over the world, approximately 15% of newborns, that is about 21 million, will require help to start breathing during the first seconds of their lives (2,19). Around 14 of the 21 million newborns will begin to breath without PPV, although these statistics overestimate results in settings with limited resources (4). Just over half of the remaining 5% (3% = 4.2 million) can achieve a satisfactory response with timely delivered PPV (20), and probably respond within an average of 16 seconds (21). Despite all the alternatives, still there are almost 2% of children in the world who may require advanced resuscitation, which are about three million newborns in extreme danger per year.
Recently proposed neonatal health research priorities include the need to explore strategies to reduce perinatal asphyxia or neonatal death through simplified neonatal resuscitation programs provided by health personnel with basic training (22). Ideally, appropriate programs should be able to be effectively implemented in resource-poor environments that too frequently do not have access to services such as electricity, oxygen sources or devices that need prior training. Proper, low-cost, portable and easy-touse neonatal devices can contribute to neonatal resuscitation efforts (4, 23), and thus providing innovative alternatives is a global challenge (24).
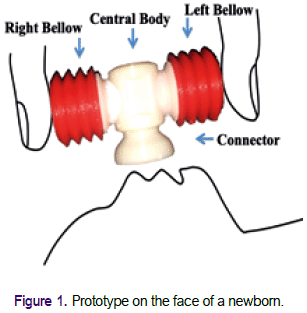
Here we present the process of evolving an innovative idea that led to the design of a portable and easy-to-use neonatal device (Figure 1). This device generates a flow of air by the pressure of its lateral bellows and can provide a respiratory stimulus to trigger the initiation of spontaneous breathing during basic neonatal resuscitation. Such a device would be particularly useful in rural and remote areas.
This study evaluated an innovative neonatal respiratory device, and it is a singular case in our country and region. The device presented in this paper obtained the Patent Title No. 8988 in 2018 (granted by the Peruvian Patent Registration Office -INDECOPI), and this title endorsed its originality after finding a paucity of similar devices in the state of the art review.
The primary objective of the study was to design, build and evaluate a lowcost portable device that could be used to stimulate spontaneous breathing, as an alternative or complement to other devices commonly used in basic neonatal resuscitation. Secondary specific objectives included 1) evolving a prototype, 2) to conduct a basic evaluation of the device in the laboratory, and 3) to conduct a non clinical evaluation of the device requesting health care personnel participation in the field.
METHODS
Study Design
We conducted an observational, descriptive, proof of concept study that in-volved the development and non-clinical assessment of a "help for the first breath" prototype.
Design process and development of the prototype in the laboratory
The overall objective is to evaluate the non-clinical performance and the acceptability by health personnel of a neonatal respiratory device produced by rapid prototyping. This prototype was designed to generate airflow and pressure by displacing air by pressing two bellows with the fingers of one hand, as shown in Figure 1. During the process, we obtained different versions of the prototype by combining a rigid centre and two bellows flexible sides. This report presents the evaluation of the prototype described in the Results section (prototype evaluated).
The prototype we have evaluated in this report has continued its self-financed development after completing the project in August 2016, and by October 2018 there was evolved a more advanced version build as a single piece without coupled structures (Data referred by the principal authors - CD and VS).
Prototype performance evaluation
We aimed to evaluate the performance of the prototype by analysing the flows and pressures generated and measured by connecting the prototype to a gas flow analyser (VT-305, Fluke Medical, United States) and a neonatal test lung.
The VT-305 measurement system has an internal processor and graphics output, and it records its measurements on a memory card.
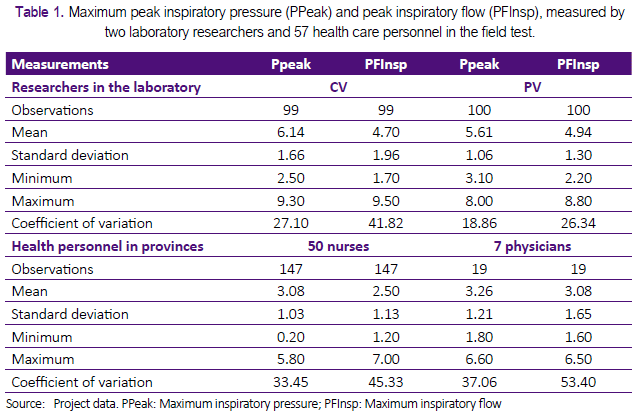
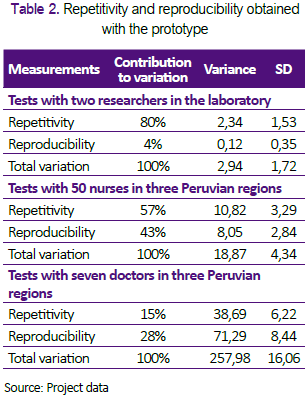
We evaluated the repeatability and reproducibility by comparing the measurements of maximum inspiratory flow (PF-Insp) and maximum inspiratory pressure (PPeak) obtained under different conditions and evaluators. We defined repeatability as the variation in the repeated measurements under similar circumstances, and reproducibility as the variation in the measurements made under changing conditions (25). The difference observed between the evaluators was presented using descriptive statistics (Table 1), which was obtained with Stata Statistical Software: Release 12.1 (College Station, TX: StataCorp LP. 2011 ®) (26). The repeatability and reproducibility were analysed in a Microsoft Excel spreadsheet, according to the available instructions for comparing different groups using analysis of variance (27) and standards for measurement procedures using a spreadsheet in MS-Excel® with instructions available online for this type of study (28).
Laboratory tests
Each test lasted 30 seconds and was performed 100 times in the laboratory by each of the two participating research neonatologists (CD and PV). During 30 seconds, each researcher generated flow and pressure with the prototype, displacing both bellows of the prototype with two fingers of one hand. We recorded each examination in the memory card of the gas flow analyser (Table 1). The researchers performed the test without predetermined frequency or pressure control. The average rate reached by the two researchers during these tests was 75 per minute (SD ± 30.3).
Field tests
We compared the reproducibility of the flows and pressures of the prototype in conditions outside the laboratory, and tests were carried out in three different regions of our country (Tarapoto, Huánuco and Ayacucho). The cities of Tarapoto, Ayacucho and Huánuco are respectively 980, 570 and 370 km away from the capital Lima. It can be reached by plane to any of them, although their indices of development are lower than the national average. They have disadvantaged socioeconomic, geographic and particular cultural conditions, along with high neonatal mortality rates.
Fifty nurses and seven doctors participated voluntarily. For the measurements, each of the participants also performed 30-second tests with the device. All received instructions on how to apply pressure on the two bellows, with no limits on force or frequency. The average rate reached by health personnel during these tests was 47 per minute (SD ± 41.6) (Table 1).
Previously, we offered these professionals a training course in neonatal resuscitation with certification to those who approved, independently of their voluntary participation in the evaluation of the prototype.
Acceptability
A survey was conducted to evaluate the acceptability of the device in 50 volunteers (39 nurses and 11 doctors). We assessed acceptability in Tarapoto and Ayacucho, due to logistical constraints. The evaluation survey was conducted at the end of the neonatal resuscitation training courses and after the device field test.
We organised three courses of neonatal resuscitation in three Peruvian cities of different regions of the country, namely Tarapoto at 250 meters above mean sea level (msnm), Huánuco at 1,800 msnm, and Huamanga at 2,746 msnm. An ad-hoc website was also developed to interact with the participants and to disseminate additional information on neonatal resuscitation (www.rcpneoperu.org, however, this link was inactivated at the end of the project). We anticipated the presence of eight to ten participants per instructor during the neonatal resuscitation courses. Before the field trips, we conducted a refreshment knowledge review for the nine participating instructors (six neonatologists and three nurses) during three months in Lima.
In addition, to accomplish this training task, two researchers (CD and VS) received training in Lexington (Kentucky, USA) almost six months before the local courses, to act as providers in the Neonatal Resuscitation Program affiliated with the American Academy of Paediatrics (AAP). One of them qualified as a Neonatal Resuscitation Instructor of the AAP (CD). A provider is the trainee that learns to perform neonatal resuscitation procedures. Instructor is the member of the health team that is a provider and who also developed and approved a course to teach providers.
A close-ended questionnaire was used to evaluate acceptability with the following alternatives:
Age, measured in years of age; Gender, classified as Male / Female; Profession, classified as Physician / Nurses; Ease of use, as Difficult use / Very simple / Not so easy; Preferences, Disposable / Reusable; Estimated price, Less than 3 USD / 3-10 USD / More than 10 USD
We hypothesised that the prototype operated manually at the discretion of the volunteer participant, with frequencies between 40 and 60 per minute, would provide a lower maximum inspiratory pressure than that provided by a self-inflatable bag (20 to 40 cm H2O). We also expected that the tidal volume generated by the prototype would be less than that offered by a self-inflatable bag (24 to 30 mL).
Ethical aspects
The ethics committee of the National Institute of Child Health, Lima, Peru, approved the research protocol and the informed consent form (Official Letter No. 00221-CEI-INSN-2015). All participants signed the informed consent before any study procedure. We included 3D scanning of neonatal faces in estimating the average measurements for facial adaptability. Parents authorised these images, signing the informed consent approved by the Institutional Ethics Committee.
RESULTS
Step I: Development of the prototype and basic evaluation
The developed prototype consists of a central body and even laterals bellows that can be of different sizes (bellows with a volume of 8 or 12 ml each pair). The prototype generates airflow when the side bellows are pressed with the fingers of one hand (Figure 1). Through an iterative trial and error process, we discard the initial designs for different structure defects and keep the most suitable configuration to carry out the tests. The prototype evaluated in this report has the side bellows coupled using a nut and screw mechanism. For the evaluations reported here, we selected the device with two bellows of 12 ml each.
Each test analysed consists of the generation of airflow and pressure with the prototype connected to the gas flow analyser, manipulating the bellows for 30 seconds. For the evaluation of the repeatability, we analysed 199 tests performed by two neonatologists in our laboratory in Lima. For the assessment of the reproducibility obtained by health personnel in the provinces, we examined 172 tests conducted by 57 volunteer participants in three Peruvian regions. We excluded trials where there were missing values in the flow or pressure measurements.
Performance: Repeatability
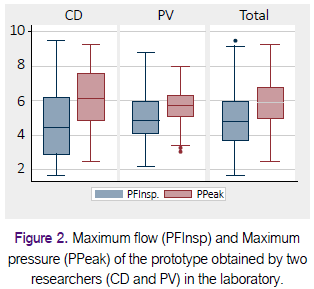
In Table 1 we present PFInsp and PPeak values obtained by two researchers (CD, PV) in the Laboratory. Figure 2 shows box diagrams that compare the measurements obtained in the laboratory by the two researchers (CD, PV). The two investigators performed 199 tests with mean PFInsp was 4.8 Lt / min (SD ± 1.7), and average PPeak was 5.9 cmH2O (SD ± 1.4).
Step II: Intermediate evaluation
Performance: Reproducibility
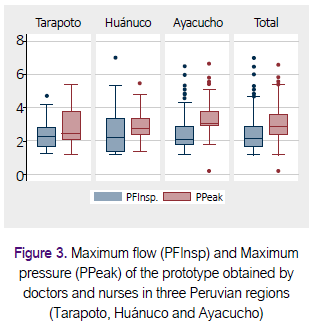
Table 2 shows a summary of the results obtained with different participants, using the Excel sheet prepared for analysis of repeatability and reproducibility (28). Figure 3 shows box diagrams to display a visual comparison of the measurements obtained by 50 nurses and seven doctors in three Peruvian regions. The 57 participants made 166 measurements with mean PFInsp was 2.6 Lt / min (SD ± 1.2), and average PPeak was 3.1 cmH2O (SD ± 1.1).
Acceptability evaluation
The acceptability survey results (n = 50) were: an average age of 43 years-old (minimum: 27 y-o, maximum 65 y-o); and a participant gender of female in 86% and male in 14%. About profession of participants, a 22% were physicians and 78%, nurses. An eighty-percent of professionals referred that the prototype have a very simple use, and 18% manifested a "not so easy" use. A 48% of participants referred a disposable preference and 52%, a reusable preference. Finally, they mentioned their preferences about an estimated price; with a 44% who preferred a price < 3 USD; 52% preferred a price between 3-10 USD; and 4% have availability to paid > 10 USD.
DISCUSSION
The optimal proportion for practising simulation scenarios during training in neonatal resuscitation is three or four students per instructor and, as a prerequisite; students must have reviewed the educational material and passed a knowledge test (29). For the courses, we had estimated the presence of eight to ten students per instructor, but we found a high requirement for training in neonatal resuscitation, which showed the magnitude of the unmet need, exceeding all our expectations. We conducted training courses in Tarapoto, Huánuco and Ayacucho, with an average of 17 students per instructor. However, this instructor:student ratio did not affect the performance in the evaluation of the prototype, as the participation in the device tests was voluntary, occurred at the end of the course and did not require previous experience.
There are administrative and regulatory restrictions that limit the development of prototypes in Peru. Prior to clinical evaluation, a device requires obtaining its sanitary registration authorized by the regulatory entity. However, the sanitary registry cannot be granted unless the clinical evaluation process has been completed. The prototype evaluated in this report was developed based on the neonatal respiratory stimulator device, which began its patent process in early 2014 at the Peruvian patent offices (INDECOPI). The holders of the file submitted to INDECOPI are two of the authors (CD and VS), and the priority date is before the search for financing for their development (patent granted, Title No. 8988). The prototype evolved after participating and obtaining funding in the contest called "Ideas Audaces" held in 2014. The prototype was developed through a rapid prototyping process in Lima, at the National Institute of Child Health, under a technical support agreement with the VEO Design Unit at the Catholic University of Peru.
The developed prototype is a low-cost device designed to offer respiratory stimulus as an alternative to tactile stimulation for the respiratory drive during basic neonatal resuscitation. This small device manually generates an air inlet that achieves an adequate current respiratory volume for a newborn, without excessive pressure. Each 12 mL bellows contributes to a total capacity of 24mL in each breath, which represents a tidal volume of 8mL/ Kg, suitable for a 3 kg child. This device may be able to generate an air intake in the newborn’s airway and stimulate the first breath through the paradoxical reflex of Head. However, the repeatability and reproducibility showed a variation of around 30% in the tests performed in Tarapoto, Huánuco and Ayacucho among doctors and nurses, which reveals the need to implement improvements in both the operator and the equipment (30).
The performance of the prototype suggests that the use of a device for respiratory stimulus like the one evaluated in this report, could help to stimulate the beginning of the breathing of newborns requiring respiratory support. The why some newborns respond to PPV may be elucidated by a physiological explanation: The paradoxical reflex of Head. This reflex generates deep inspiration against pulmonary inflation and is related to the effects of initial lung ventilation after birth (31). This prototype can probably decrease the PPV requirement through this physiological effect, as well as may reduce PPV complications and sequelae. The universal use of our device could help some of the 4.2 million newborns who currently require PPV and who may not receive it promptly or adequately. We estimate that the universal use of our device could help at least 30% of newborns who need help to begin to breath. Therefore, it has the potential to prevent about 1.2 million deaths or disabilities per year, that is, one third of the 3.6 million neonatal deaths reported by Lawn et al (2010) (32).
The first breath stimulator we have developed is a low-cost device, designed to offer respiratory stimuli as an alternative to tactile stimulation for an early respiratory drive. This small device manually generates an air inlet that achieves an adequate current respiratory volume for a newborn, without excessive pressure. In any case, the analysis of repeatability and reproducibility shows that the variation of around 30% shows the need for performance improvements in both the operator, the equipment and the methods evaluated (30).
The performance of our prototype is not yet uniform outside the laboratory. Variations in their performance in the fieldwork show that there is still a need to improve the design and the instructions for use by health care personnel in remote areas. Likewise, it is necessary to evaluate their clinical efficacy and safety performance through an adequate experimental design.
We have not found studies evaluating prototypes similar to ours. However, in 2017, the results of the evaluation of the performance and acceptability of a self-inflating neonatal bag for vertical use were published compared to the conventional design for use in the standard transverse position (33-35). The first study assessed the performance of the devices analysing videos of participants ventilating a manikin using an upright device compared to the standard device (33). The second trial found that the vertical device offered higher volumes and lower mask leakage compared to the standard in a manikin model (34). The third study developed a trial in Tanzania to compare both devices, and it founds relevant results favouring the upright device (35). An appropriate sequence for efficacy and safety assessment in medical devices requires to progress from the evaluation in the laboratory to the evaluation in manikins and humans described, as described in this section.
The global increase in support for innovation and scientific development raises the need for Peruvian public institutions to remain at the forefront of this scenario, avoiding unnecessary, lengthy and cumbersome bureaucratic requirements, developing internal regulations that actually promote the surge of use-ful innovation groups and the efficient approval process of intellectual property rights for innovative medical devices.
The health personnel who participated in the study were not a probabilistic or representative sample of any health institution. On the other hand, we realise that we assess subjectively the ease of use and estimated price provided by the health personnel. Notwithstanding, it is also essential to acknowledge that both performance and acceptability assessment requires further research in a controlled clinical study.
We present the first breath trigger for neonatal respiratory stimulus, as an innovative alternative that achieved its proof of concept, and is now ready for its clinical assessment through adequate designs.
ACKNOWLEDGEMENTS
The Neonatal Research Group UNMSM is composed of Peruvian neonatologists and nurses devoted to the care of newborns and affiliated to the Universidad Nacional Mayor de San Marcos. Many of them work at the Instituto Nacional de Salud del NinÞo (INSN) in Lima, Peru, and perform clinical care activities, clinical teaching and scientific research. The authors are grateful for their participation and support to Enrique Gomez from University of Kentucky, Lisset Aguilar, Karen Rios, Luz Gallardo, Rosa de la Cruz, Nancy Arce, Juana La Rosa, Carlos Lomparte, Percy Martinez, Irene Valencia, Nadia García, and all staff from Neonatal Unit at the INSN. We also acknowledge Dr Luis Cifuentes, Chief of Experimental Surgical Unit at INSN, and all his staff for his invaluable support.
COMPLEMENTARY MATERIAL
The first minimum value product is shown in a video, available in https://figshare.com/s/c259dd8faa6bef357fd5. Additionally, the performance of the prototype through a gas flow analyser and a neonatal test lung is showed in https://figshare.com/s/2c05389964528246ab93.
REFERENCES
1. World Health Organization. Guidelines on Basic Newborn Resuscitation. Geneva: World Health Organization; 2012. [ Links ]
2. Ersdal HL, Mduma E, Svensen E, Perlman JM. Early initiation of basic resuscitation interventions including face mask ventilation may reduce birth asphyxia related mortality in low-income countries: a prospective descriptive observational study. Resuscitation. 2012;83:869-73. DOI: http://dx.doi.org/10.1016/j.resuscitation.2011.12.011. [ Links ]
3. Umphrey L, Breindahl M, Brown A, Saugstad OD, Thio M, Trevisanuto D, et al. When Helping Babies Breathe Is Not Enough: Designing a Novel, Mid-Level Neonatal Resuscitation Algorithm for Medecins Sans Frontieres Field Teams Working in Low-Resource Hospital Settings. Neonatology. 2018;114(2):112-23. DOI: http://dx.doi.org/10.1159/000486705. [ Links ]
4. Ersdal HL, Singhal N. Resuscitation in resourcelimited settings. Semin Fetal Neonatal Med. 2013;18(6):373-8. DOI: http://dx.doi.org/10.1016/j.siny.2013.07.001. [ Links ]
5. Bhutta ZA, Das JK, Bahl R, Lawn JE, Salam RA, Paul VK, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? The Lancet. 2014;384(9940):347-70. DOI: http://dx.doi.org/10.1016/S0140-6736(14)60792-3. [ Links ]
6. Dickson KE, Simen-Kapeu A, Kinney MV, Huicho L, Vesel L, Lackritz E, et al. Every Newborn: health-systems bottlenecks and strategies to accelerate scaleup in countries. Lancet. 2014;384:438-54. DOI: http://dx.doi.org/10.1016/S0140-6736(14)60582-1. [ Links ]
7. Niermeyer S. From the Neonatal Resuscitation Program to Helping Babies Breathe: Global impact of educational programs in neonatal resuscitation. Semin Fetal Neonatal Med. 2015;20(5):300-8. DOI: http://dx.doi.org/10.1016/j.siny.2015.06.005. [ Links ]
8. Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, et al. Part 13: Neonatal Resuscitation 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18):S543-S60. DOI: http://dx.doi.org/10.1161/cir.0000000000000267. [ Links ]
9. Ariawan I, Agustini M, Seamans Y, Tsu V, Kosim MS. Choosing the appropriate neonatal resuscitation device for village midwives. J Perinatol. 2011;31(10):664-70. DOI: http://dx.doi.org/10.1038/jp.2011.7. [ Links ]
10. Kim YM, Ansari N, Kols A, Tappis H, Currie S, Zainullah P, et al. Assessing the capacity for newborn resuscitation and factors associated with providers’ knowledge and skills: a cross-sectional study in Afghanistan. BMC Pediatr. 2013;13:140. DOI: http://dx.doi.org/10.1186/1471-2431-13-140.
11. Khoury A, Hugonnot S, Cossus J, De Luca A, Desmettre T, Sall FS, et al. From mouth-to-mouth to bag-valve-mask ventilation: evolution and characteristics of actual devices--a review of the literature. BioMed research international. 2014;2014:762053. DOI: 10.1155/2014/762053. [ Links ]
12. Coffey PS, Saxon EA, Narayanan I, DiBlasi RM. Performance and Acceptability of Two Self-Inflating Bag-Mask Neonatal Resuscitator Designs. Respir Care. 2015;60(9):1227-37. DOI: 10.4187/respcare.03867. [ Links ]
13. Kattwinkel J, Perlman JM, Aziz K, Colby C, Fairchild K, Gallagher J, et al. Neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2010;126(5):e1400-13. DOI: http://dx.doi.org/10.1542/peds.2010-2972E. [ Links ]
14. Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology. 2008;94:176-82. DOI: http://dx.doi.org/10.1159/000143397. [ Links ]
15. Vento M. Oxygen supplementation in the neonatal period: changing the paradigm. Neonatology. 2014;105:323-31. DOI: http://dx.doi.org/10.1159/000360646. [ Links ]
16. Shimabuku R, Ota A, Pereyra S, Véliz B, Paz E, Nakachi G, et al. Hyperoxia with 100% oxygen following hypoxia-ischemia increases brain damage in newborn rats. Biol Neonate. 2005;88:168-71. DOI: http://dx.doi.org/10.1159/000086206. [ Links ]
17. Benavides M, Shimabuku R, Ota A, Pereyra S, Delgado C, Sánchez V, et al. Hiperoxia por dos horas produce daño morfológico cerebral luego de asfixia neonatal experimental. An Fac Med (Perú). 2013;74(4):273-7. http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1025-55832013000400002&lng=es&nrm=iso.
18. Saugstad OD. The oxygen paradox in the newborn: keep oxygen at normal levels. The Journal of Pediatrics. 2013;163:934-5. DOI: http://dx.doi.org/10.1016/j.jpeds.2013.06.003. [ Links ]
19. Lee AC, Cousens S, Wall SN, Niermeyer S, Darmstadt GL, Carlo WA, et al. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Public Health. 2011;11:S12. DOI: http://dx.doi.org/10.1186/1471-2458-11-S3-S12. [ Links ]
20. Wyllie J, Perlman JM, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: Neonatal resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2015;95:e169-201. DOI: http://dx.doi.org/10.1016/j.resuscitation.2015.07.045. [ Links ]
21. Skare C, Boldingh AM, Nakstad B, Calisch TE, Niles DE, Nadkarni VM, et al. Ventilation fraction during the first 30s of neonatal resuscitation. Resuscitation. 2016;107:25-30. DOI: http://dx.doi.org/10.1016/j.resuscitation.2016.07.231. [ Links ]
22. Yoshida S, Rudan I, Lawn JE, Wall S, Souza JP, Martines J, et al. Newborn health research priorities beyond 2015. Lancet. 2014;384:e27-9. DOI: http://dx.doi.org/10.1016/S0140-6736(14)60263-4. [ Links ]
23. Deakins K. Manual ventilation during resuscitation: does device matter? Respir Care. 2012;57:655-6. DOI: http://dx.doi.org/10.4187/respcare.01779. [ Links ]
24. Darmstadt GL, Oot DA, Lawn JE. Newborn survival: changing the trajectory over the next deCVe. Health Policy Plan. 2012;27:iii1–iii5. DOI: http://dx.doi.org/10.1093/heapol/czs054. [ Links ]
25. Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol. 2008;31(4):466-75. DOI: http://dx.doi.org/10.1002/uog.5256. [ Links ]
26. StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP. 2011. [ Links ]
27. Botero Arbeláez M, Arbeláez Salazar O, Mendoza Vargas JA. Método ANOVA utilizado para realizar el estudio de repetibilidad y reproducibilidad dentro del control de calidad de un sistema de medición. Scientia. 2007;1(37):533-7. DOI: http://dx.doi.org/10.22517/23447214.4181. [ Links ]
28. Muelaner J. Gage Repeatability and Reproducibility (Gage R&R) in an Excel Spreadsheet 2016 [cited 2016 October 5th, 2016]. Available from: https://www.muelaner.com/quality-assurance/gage-r-andr-excel/. [ Links ]
29. Zaichkin J, Kattwinkel J, Weiner GM, Association AH, Pediatrics AAo. Instructor manual for neonatal resuscitation: American Heart Association; 2011. [ Links ]
30. Portuondo Paisan Y, Portuondo Moret J. La repetibilidad y reproducibilidad en el aseguramiento de la calidad de los procesos de medición. Tecnología química. 2016;30(2):117-21. 2224-6185. [ Links ]
31. te Pas AB, Davis PG, Hooper SB, Morley CJ. From liquid to air: breathing after birth. J Pediatr. 2008;152(5):607-11. DOI: http://dx.doi.org/10.1016/j.jpeds.2007.10.041. [ Links ]
32. Lawn JE, Kerber K, Enweronu-Laryea C, Cousens S. 3.6 Million Neonatal Deaths—What Is Progressing and What Is Not? Semin Perinatol. 2010;34:371-86. DOI: http://dx.doi.org/10.1053/j.semperi.2010.09.011. [ Links ]
33. Narayanan I, Mendhi M, Bansil P, Coffey PS. Evaluation of Simulated Ventilation Techniques With the Upright and Conventional Self-Inflating Neonatal Resuscitators. Respir Care. 2017;62(11):1428-36. DOI: 10.4187/respcare.05328 [ Links ]
34. Thallinger M, Ersdal HL, Morley C, Purington C, Gomo O, Mduma E, et al. Neonatal ventilation with a manikin model and two novel PEEP valves without an external gas source. Arch Dis Child Fetal Neonatal Ed. 2017;102(3):F208-f13. DOI: 10.1136/archdischild-2016-310955. [ Links ]
35. Thallinger M, Ersdal HL, Francis F, Yeconia A, Mduma E, Kidanto H, et al. Born not breathing: A randomised trial comparing two self-inflating bag-masks during newborn resuscitation in Tanzania. Resuscitation. 2017;116:66-72. DOI: 10.1016/j.resuscitation.2017.04.012. [ Links ]
Conflictos de interés: CD y VS son propietarios de la patente registrada en el Sistema Peruano de Invenciones y Registros de Propiedad Intelectual de INDECOPI.
Fuente de financiamiento: El gobierno peruano, en conjunto con el Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica (Contrato Nº 053-2014-FONDECYT) compartieron el financiamiento, en asociación con el gobierno canadiense y Grand Challenges Canada (GCC, Grant S7 0690-01-10). Se obtuvo este financiamiento conjunto a través de un concurso publico denominado "Ideas Audaces 2014" en Perú y "Stars in Global Health - Round 7" en Canadá.
Contribuciones de autoría: CD y VS participaron en la concepción del estudio, la recopilación, el análisis e interpretación de los datos y la redacción del manuscrito. PV y RS participaron en la recopilación, análisis e interpretación de los datos. Todos los autores realizaron la revisión crítica del artículo, hicieron contribuciones a su redacción y aprobaron la versión final del artículo.
Correspondencia:
Carlos A. Delgado
cdelgadob1@unmsm.edu.pe
Recibido: 17 de abril 2019
Aceptado: 12 de agosto 2019
Publicación en línea: 30 de setiembre 2019














