INTRODUCTION
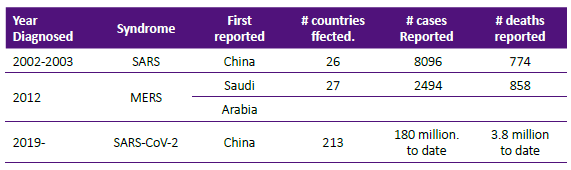
Although the origin of viruses is unclear, they appeared billions of years before humans evolved on earth. The viruses are not considered living organisms and do not have their own metabolism. They depend on the cell host to survive, multiply and spread. In doing that, they disrupt cell function and engender disease. Scientists have classified viruses by their shape, their cell host or their genetic code. Of interest to this article is the genetic code classification, viruses can be RNA or DNA viruses. The SARS-CoV-2 and its family of coronavirus are classified as RNA who belong to the family Coronaviridae. Four are known to cause seasonal community acquired upper respiratory tract infections: 229E, NL63, OC43 and HKU1.Besides the actual pandemic virus, there were two earlier SARS viruses responsible for serious although limited outbreaks as described in Table 1.
DEVELOPMENT OF THE TOPIC
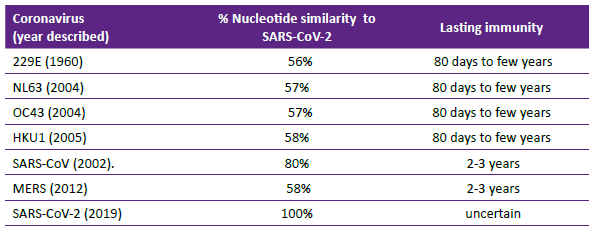
In December 2019, the first cases of rapidly spreading pneumonia were reported from Wuhan City, China. Its most likely origin was zoonotic, hypothesized to have started from 'wet' markets common in China, with bats and pangolins considered the likely reservoirs. However, its origin is still being investigated. The virus was promptly sequenced by Chinese scientists and data made available in early January 2021 1. The development of diagnostic tests and vaccines were enhanced by this knowledge. SARS-CoV-2 nucleotides similarity to other coronaviruses and possible length of immunity is shown in Table 2. Reinfection is not uncommon with coronaviruses; it is uncertain if it due to short-lived immunity or infection with variants of the same virus or probably contribution of both mechanisms 2. Whether pre-existing immunity to seasonal coronaviruses offer cross protection against SARS-CoV-2 is an important question whose answer is unknown. The structure of the SARS-CoV-2 virion is known to be a spherical, enveloped virus with three structural proteins: the spike glycoprotein, the membrane protein and the envelope protein. The nucleocapsid protein is associated with the membrane protein.
The spike glycoprotein allows the SARS-CoV-2 to attach to cells that have angiotensin converting enzyme 2 (ACE2) receptors (lung, intestine and others). Humoral and cellular immunity are of primary importance to control the infection. If the innate anti-viral pathway is not enough to stop the infection, the viral RNA is released into the cell and a cascade of events ensues culminating in the assembly of daughter virions. If the infected human is able to stimulate an immune response (natural or due to vaccination), the B cells will produce neutralizing antibodies targeting the spike and the receptor binding domain. Another important mechanism of defense is the activation of Th cells and cytotoxic lymphocytes stimulating production of cytokines which will recognize and kill infected cells. Both B and T cells have immunological memory which last at least 6 months 3. Although it is expected to last 12 months or more.
It is important to understand that the correlates of protection against SARS-CoV-2 are unknown as such the levels of antibodies or role of T cells in suppressing the infection have not been yet completely elucidated.
Clinical manifestations
After the incubation period, usually of 5 days, with a range of 1-14 days, symptoms appear, some subjects remain asymptomatic (see below). The most common symptoms are fever (88%), cough (68%), fatigue (38%) and in decreasing percentages: shortness of breath, sore throat, muscle pain. Some may experience loss of the sense of smell, nausea, vomiting and diarrhea. This wide variety of clinical disease phenotypes suggest that many clinical factors are associated with severity, such as age, gender and ethnicity of the individuals. Existing comorbidities like diabetes, obesity, smoking status play a role. And importantly, the immune response (neutralizing antibody and CD4/CD8 responses) are found to play a significant function 4.
Asymptomatic infection
Quantifying the proportion of symptom-free individuals relative to symptomatic subjects is difficult and not unique to the SARS-CoV-2 infection. However, the range of clinical manifestations observed varies from no symptoms, to mild illness, severe illness and death in this particular viral disease is striking. A systematic review of 61 studies showed about a third of more than 1.8 million who tested positive by nasal swab polymerase chain reaction (PCR) or by antibody testing, had no symptoms at the time of testing. And about 75% of this group remained free of symptoms for the duration of follow up 5. It is important to stress that asymptomatic individuals are capable of transmitting infection that becomes symptomatic as shown in an epidemiologic study in China 6. The vaccine is capable to reduce asymptomatic infections 7.
Diagnosis of SARS-CoV-2 infection
The diagnosis is made by detection of viral RNA in nasal swabs or saliva specimens by polymerase chain reaction (PCR) or by enzyme-linked immunosorbent assay measuring IgG antibody to the receptor binding domain of the spike protein. Antigen testing is available but has less sensitivity and is not the preferred method. Neutralizing antibodies are complex and expensive tests and not necessary for diagnosis of SARS-CoV-2 infection. The dynamics of virus-induced antibodies require long term studies.
Vaccines
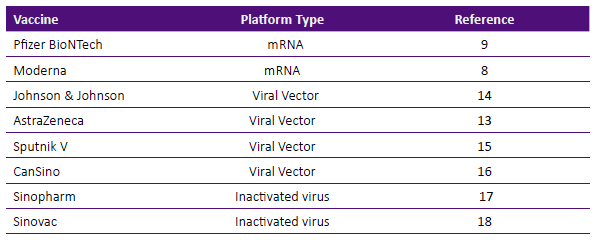
Scientists used known vaccine platforms like inactivated virus (whole non- viable virus) as well as novel systems, not previously tested in human such as synthetically produced messenger RNA (mRNA) or vaccines that use non-replicating adenoviral vectors. At this time more than 75 vaccines are in development and 12 have been authorized for use in different countries. In the United States of America, three vaccines received emergency use authorization from the U. S. Food and Drug Administration: two mRNA vaccines: Moderna and Pfizer-BioNTech and one adenovirus vector product: Johnson & Johnson/Janssen. Besides the three products, other vaccines are in use in other parts of the world as depicted in Table 3 and will be briefly described.
mRNA vaccines
Both products, Moderna and Pfizer- BioNTech utilize the SARS-CoV-2 spike protein as their antigen. They are synthetically produced mRNA encoding an area of the spike protein, they contain a lipid particle. Once injected in muscle, the mRNA is conducive to the formation of viral antigen provoking antibody formation. The vaccines are injectable, 3 to 4 weeks apart and in phase 3 clinical trials have demonstrated a 95% efficacy as compared to placebo 8,9. There were side effects but most were not severe and temporary. Anaphylactic reactions were reported at a rate of one in about one million of immunized subjects 10,11. In Israel, a population observational study of more than 500,000 immunized versus a similar number of non-immunized, confirmed a 94% protection 12.
Viral Vector Vaccines
As shown in Table 3, at least four vaccines using an inactivated viral vector system have been tested in humans. AstraZeneca vaccine uses a chimpanzee- derived adenovirus (ChAdOx1), the Johnson & Johnson/Janssen vaccine uses a recombinant adenovirus (Ad26), the Russian vaccine Sputnik V uses recombinant Ad26 and Ad5 and CanSino uses recombinant Ad5.
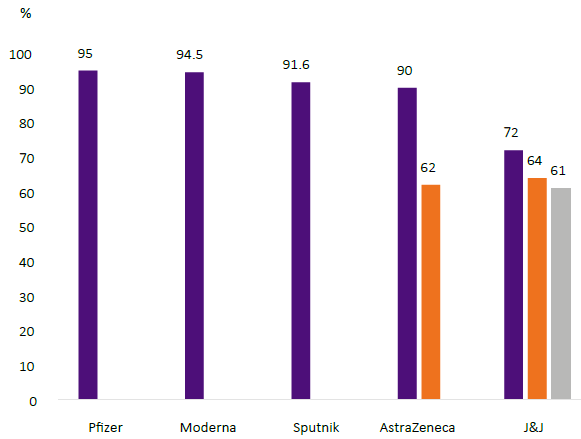
The phase 3 clinical trial efficacy results are shown in Figure 1 (8,9,13,14,15). AstraZeneca shows two rates because a group in the study received half a dose followed by a full dose, the low-dose initial group had an efficacy rate of 90%, while the group who received two full doses had 62%. Sputnik V is administered in two injections spaced 4 weeks apart, an efficacy rate of 91.6% was reported. In recent communication in the press, we learned of Sputnik V 'light' or one-dose vaccination was successful.
The Johnson & Johnson/Janssen vaccine phase 3 clinical trials rendered a 67% efficacy rate after single dose administration.
In a phase 2 trial, CanSino vaccine 16, was reported to induce significant immune responses after a single immunization with a dose 5x1010. Phase 3 clinical trial is not available in the scientific literature.
Inactivated Vaccines
Sinopharm and Sinovac vaccines 17,18 were manufactured using traditional methods of cell culture, chemically inactivated ad adjuvanted with alum. The efficacy rates of phase 3 clinical trials have not been published but reported by their manufacturers as to be 79% for Sinopharm, although recently the efficacy has been questioned because of large out- breaks in countries where Sinopharm vaccine was widely used. Sinovac was 50.3% effective in a Brazilian trial and 83.5% in Saudi Arabia. Sinopharm vaccine recently received emergency use authorization by the World Health Organization.
Viral Variants
Viruses have the ability to mutate, this characteristic makes them able to evade the immune system and continue multiplying. This attribute is not unique to SARS-CoV-2 but to just about all existing viruses. Certain viruses like influenza are notorious for escaping and not allowing a universal vaccine.
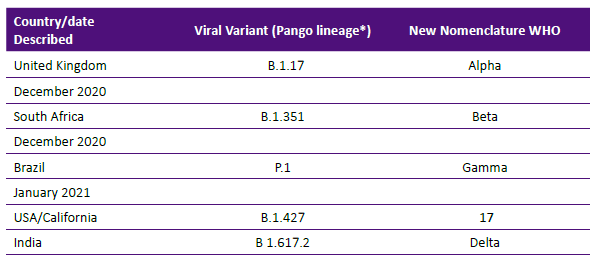
The current vaccines against SARS- CoV-2 were formulated against the spike zone and the receptor binding domain (RBD) zone of the virus and Table 4 shows the reported variants of concern.
The variants have two worrisome abilities: a) evade immunity from vaccine or infection with original SARS-CoV-2 virus causing illness and b) increase affinity to ACE-2 therefore allowing rapid viral spread. Some variants like the ones described in South Africa and India, combine both types of mutations and are of particular concern. For example, during a phase 3 trial in South Africa, the Astra- Zeneca vaccine induced antibody was not able to neutralize the variant virus and its reduction of potency was described as -86% 19, The variant from the United Kingdom is more transmissible but does not evade vaccine-induced antibody in significant amount.
In addition, there are reports of individuals fully immunized who have breakthrough infections despite robust vaccine-induced antibody. Viral sequencing in these cases showed the mutations of concern that allow neutralizing antibo- dy-viral escape 20 and reduction of vaccine efficacy 21.
Adverse Events
Besides the anaphylactic reactions observed after the mRNA vaccines 10,11, the risk of serious adverse events is low after immunizing more than 400 million people 22. However, cases of immune thrombocytopenia and bleeding without thrombosis after both mRNA vaccines were reported 23. Similar cases after the AstraZeneca vaccine and the Johnson & Johnson vaccine have been reported in different parts of the world were described 24), several of the thromboses occurred at unusual sites, like cerebral venous sinus thrombosis or pulmonary emboli or acute arterial thromboses. Treatment with intravenous immune globulin and high-dose glucocorticoids have improved the significant thrombocytopenia observed in these cases. Early recognition is paramount.
Pericarditis and myocarditis has been described in young people after receipt of mRNA vaccines, it is a rare syndrome and individuals affected recovered without known sequelae 25,26.
CONCLUSION
The pandemic generated by SARS-CoV-2 infection is still ongoing and no herd immunity has been accomplished. In places where 50% or more of the population has been immunized, the rate of infections and covid-related deaths are substantially decreasing and the economy is recovering. Certain regions where the vaccine use is lagging are at higher risk, not only of infection but of generating new viral variants. At this time there are no variants of high consequence but one or more could be initiated rendering null the efforts to control the infection. Despite some serious side effects in some individuals, the protection afforded by the vaccine outweighs the risks and is highly encouraged.