INTRODUCTION
Guinea pigs are domestic mammals, hystricomorph rodents of the Caviidae family, autochthonous to the Andes region (Perú, Ecuador, Bolivia, and Colombia) (Lord et al., 2020). They had a close relationship with the pre-Inca people as a source of food high in protein and low in fat and associated with traditions that persist to this day (Avilés et al., 2014a). These are emblematic animals for their diverse utility. In addition to the role of a pet, guinea pigs are used as an animal model for biomedical research (Dyson et al., 2012; Matsuzawa et al., 2018; Morrison et al., 2018; Sterusky et al., 2020). These mammals also play an important role as a high-quality food source (Lammers et al., 2009; Sánchez-Macías et al., 2018), contributing to the family income of several Latin American countries (Avilés et al., 2014a), and as a source of excellent quality compost (Aliaga et al., 2009).
In Ecuador and other Latin American countries, there are three production systems of guinea pigs: family-traditional, family-commercial, and commercial, being the first the most prominent (Avilés et al., 2014b). In general, these production systems manage two genetic groups: native or improved. The former are small, rustic animals and undemanding in terms of food quality, and the latter are considerably larger and more productive and are the result of a process of genetic improvement (Chauca, 1995).
Guinea pigs are non-seasonal breeders and spontaneous ovulators, with a gestation length allowing several calvings by year and several offspring per calving (Lammers et al., 2009). The length of the estrous cycle is approximately 16 days (13-22 days) (Shi et al., 1999; Shomer et al., 2015). Ovulation rate is 2.8 ± 0.9 and 3.6 ± 0.9 for Peru and Andean breeds (Araníbar and Echevarría, 2014). The gestation period is an average 68-day length (59-72 days) (Shomer et al., 2015). Litter size varied according to the genetic group: 2.4 ± 0.22 in two local native strains (Azuay y Cañar) and 3.4 ± 0.32 in Peru breed females (Cedillo and Quizhpi, 2017), however litter size varies according to parity (Fernández, 2019). The weight of the ovary varied throughout the estrous cycle between 90 and 125 mg, and number of follicles in both ovaries averaged 101 ± 9 (60-105 follicles) (Labhsetwar and Diamond, 1970). Follicular growth occurs in two waves (Bland, 1980; Hamilton and Tam, 1990); the first ending in atresia between days 9-12 and the second wave culminating in ovulation at the end of the estrous cycle (Hamilton and Tam, 1990).
Characterizing the reproductive aspects of both genetic groups is an important step for further plans on in vitro production and cryopreservation of guinea pig embryos. Information on morphological and histological features of native and improved guinea pig ovaries is scarce or non-existent. Accordingly, this study aimed to characterize morpho- logically and histologically the ovary of two genetic groups, native and improved, of gui- nea pigs from Latin America Andes.
MATERIALS AND METHODS
Study Location and Animal Management
This research was conducted at the Faculty of Agricultural Sciences, University of Cuenca, Ecuador. Forty adult female gui- nea pigs were used, 20 from native («Azuay and Cañar» native genetic lines) and 20 from improved genetic groups («Auquicuy and Macabeo» Ecuadorian lines), clinically healthy, 1 to 3 calving, with a minimum adult weight of at least 80%, reproductively active and not pregnant.
Animals were handled according to procedures approved by the Veterinary Science Committee, Faculty of Agricultural Sciences, University of Cuenca, and the research was performed following chapter 7.8 of the Terrestrial Animal Health Code - 2019© OIE (07/08/2019), regarding the protection of animals used in scientific experiments. All animals were housed under uniform handling and feeding conditions. They were fasted for a period of 12-14 h overnight and then were slaughtered by cervical neck dislocation, one of the euthanasia methods approved by the American Veterinary Medical Association (AVMA, 2020).
Vaginal Cytology
Immediately after slaughter, vaginal smears were obtained to determine the stage of the estrous cycle following the criteria described by Stockard and Papanicolau (1917) for Cavia porcellus. Smears were taken from the proximal third of the vaginal epithelium by inserting a wet sterile swab roughly 2 cm into the vagina and twice rotating it against the vaginal wall. Each swab was rotated onto a microscope slide, and each smear was air-dried and fixed with ethyl al- cohol to preserve cellular morphology. Vaginal smears were stained with Wright® staining (JQWRG-1 K0-00, Quimical, Ecuador), and observed under a microscope (Olympus CX31, Germany) at x10, 20 and 40 magnifications.
Ovary Collection and Evaluation
To account for the effect of anatomical location, the right or left ovaries of the first guinea pig incorporated into the study was randomly assigned to study histological and morphological characteristics respectively and then alternated successively in subsequent animals so that each aspect studied in each genetic group consisted of 10 left and 10 right ovaries. Accordingly, the ovaries selected for morphology and oocyte micrometry were immersed in physiological solution at 37 ºC and transported to the laboratory for weighing, measuring, and quantifying the follicles visi- ble on the ovarian surface. Ovaries to des- cribe the follicular population were cut in half and immersed in refrigerated Bouin fixative solution for six hours (Osman, 1985).
The major and minor diameter of each ovary was measured with a digital caliper and weighed on an analytical balance (Boeco, BAS 31plus, Germany). All follicles located on the ovarian surface were counted. Immediately, cumulus-oocyte complexes (COCs) were collected by the slicing method and rinsed three times in synthetic oviductal fluid (SOF). Cumulus-oocytes complexes (improved: n=249; native: n=259) were identified and classified into three categories:
A) five or more compact layers of cumulus cells surrounding the oocyte and cytoplasm with homogeneous granulation (improved: n=71; native: n=73); B) less than five compact layers of cumulus cells and totally or partially homogeneous cytoplasm (improved: n=87; native: n=101); and C) oocytes partially or totally free of cumulus cells and/or with expanded cumulus cells and/or mostly heterogeneous cytoplasm (improved: n=91; native: n=85). Cumulus-oocytes complexes were exposed to hyaluronidase (Sigma- Aldrich) (1 mg/ml) for 2-5 min and the cumulus cells removed by gentle pipetting. Oocytes were photographed with a digital camera (Exelis AU-60 0-HD, USA) coupled to a phase contrast microscope at x100 magnification (Olympus CX31, Japan). The thickness of the zona pellucida and oocyte diameter (excluding zona pellucida) were measured using the AmScope (v. 3.7) soft- ware.
Histological Processing
After fixation, the ovaries were embedded in paraffin, cut into 5 µm sections, and five sections mounted on each microscope slide. Serial sections mounted on each microscope slice were separated from each other by 50 µm of ovarian tissue. After deparaffinization with xylene and rehydration through descending grades of alcohol, tissue specimens were stained with hematoxylin and eosin.
To avoid enumerating the same follicle twice or more times, only antral follicles showing an oocyte inside, and secondary and primary follicles with a visible nucleolus were considered. Primordial follicles were quantified even in the absence of a nucleolus but with an easily identifiable nuclear membrane (Bosch et al., 2004). Primordial follicles were identified as structures having one layer of flattened granulosa cells surrounding the oocyte (the presence of at least one flattened cell within the layer was sufficient to include the follicle in this category), primary follicles were those with one layer of cuboidal granulosa cells, secondary follicles had two or more layers of cuboidal granulosa cells, and antral follicles had a fluid-filled antral cavity inside (Hernandez-Fonseca et al., 2005). The gonadosomatic index was determined (GSI = [ovary weight / body weight] × 100). The follicle count reported in this study corresponds to the average number per ovary.
Statistical Analysis
Variables showing a skewed distribution (as determined by the Shapiro-Wilks test) were Log10 transformed. The effect of the genetic group on morphological charac- teristics of ovaries (size and weight) and oocytes (zona pellucida thickness and oocyte diameter), and follicle categories (histological sections) were analyzed by analysis of variance using the GLM (General Linear Model) procedure of SAS (Statistical Analysis System, 2012; version 9.4). Ovarian location (right or left), estrous cycle phase (diestrus and proestrus), and the genetic group × estrous cycle phase interaction were considered as independent variables. In oocyte morphometry, in addition to the genetic group, the oocyte category was considered as an independent variable. Statistical significance was considered as p<0.05, and p values between 0.051 and 0.1 were considered as tendency. Values are expressed in mean ± standard error.
RESULTS
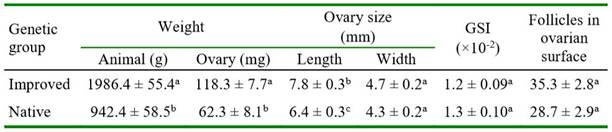
As expected, the guinea pigs of the improved genetic group had greater body weight and heavier ovaries than the native guinea pigs (p<0.0001). The ovary length in improved animals was statistically greater but the ovary width was quite similar between genetic groups. The GSI did not differ between groups. The follicle count in the ovarian surface was greater but not significant in improved than in native guinea pigs (Table 1).
In native guinea pigs, the ovary length and width, the GSI, and the follicular count were similar in diestrus and proestrus (Table 2). In improved guinea pigs, ovarian weight was greater in diestrus than in proestrus (p=0.0632), while the other variables were similar between phases of the estrous cycle. Ovarian weight was greater in both phases of the estrous cycle in improved than in native guinea pigs (p<0.0001). The ovary length and width were significantly greater only in diestrus of improved guinea pigs compared with the same phase of the estrous cycle in the native group (Table 2).
The number of primordial, primary, secondary, and total follicles did not differ between genetic groups. However, there was a significantly greater number of antral follicles in improved than in native guinea pigs (Table 3). In improved guinea pigs, the number of all categories of follicles did not differ between both phases of the estrous cycle (Table 4). In native guinea pigs, there was a greater number of primordial (p=0.0770, primary (p=0.0091), and total (p=0.0222) follicles in diestrus than in proestrus. Greater number of primary follicles was observed in proestrus in improved than in native guinea pigs (p=0.0802). Also, a significantly greater number of antral follicles was observed in proestrus (p=0.0389) in improved than in native guinea pigs. There was no effect of ovarian location (right or left) on follicle count in either follicle category in both groups of guinea pigs (data not shown).
Table 2.Ovary weight, gonadosomatic index (GSI), ovary size and number of follicles in the ovarian surface in native and improved guinea pigs, according to the phase of estrous cycle
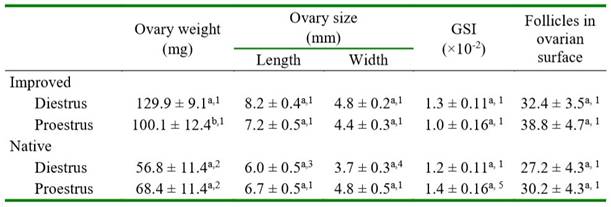
Different letters in the same column for each genetic group differ (a-b p= 0.0632)
Different numbers in the column between the same estrous cycle phase differ (1-2 p<0.0001; 1-3 p=0.0009; 1-4 p=0.0115; 1-5 p=0.0516)
Table 3. Number of follicles of native and improved guinea pig ovaries

Different letters in the same column differ (a-b p=0.0158)
Table 4. Number of follicles in native and improved guinea pig ovaries according to the phase of estrous cycle
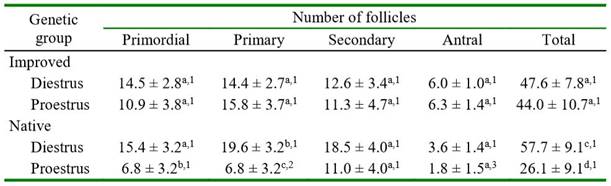
Different letters in the same column for each genetic group differ (a-b p=0.0770; b-c p=0.0091; c-d p=0.0222)
Different numbers in the column between the same estrous cycle phase differ (1-2 p=0.0802; 1-3 p=0.0389)
The proportion of A, B, and C oocytes was similar in improved (28.5, 34.9, and 36.5% respectively) and native (28.1, 38.9, and 32.8% respectively) guinea pigs. The thickness of zona pellucida (14.8 ± 0.18 and 15.1 ± 0.18 µm for improved and native gui- nea pigs, respectively) and oocyte diameter (78.6 ± 0.32 and 78.6 ± 0.31 µm for improved and native guinea pigs, respectively) were quite similar between genetic groups. The oocyte diameter was statistically similar between oocyte categories in both genetic groups (data not shown). The thickness of the zona pellucida was significantly greater in oocytes of category A than in oocytes of categories B and C in both improved (15.9 ± 0.34a, 14.3 ± 0.30b and 14.1 ± 0.31b respectively; a-b p<0.001) and native (15.6 ± 0.33c,e, 14.7 ± 0.30d,e, and 15.0 ± 0.31e, respectively; c-d p<0.05) guinea pigs.
DISCUSIÓN
Statistical analysis showed marked differences in terms of body and ovarian weight and ovarian size between improved and native genetic groups. In general, the morphometric and histological traits studied were quite similar. In some cases, however, variation prevented the numerical differences, which were sometimes notable, from being statistical as well. The zootechnical characteristics of improved and native gui- nea pigs have been previously described (Chauca, 1995; Aliaga et al., 2009). The improved guinea pigs in this study resulted from many years of genetic improvement in traits related to meat production. The native variety, consisting of smaller and more rustic animals, was not subjected to systematic genetic selection, so their body weight was slightly less than half that of the former. The weights of 90-day-old nulliparous guinea pigs from the same native population and improved guinea pigs of a different genetic line than the used in this study were reported a 542.0 ± 17.1 and 874.2 ± 27.9 g, respectively (Cedillo and Quizhpi, 2017).
Body and ovarian weights were about twice as great in females from improved than from native guinea pigs. As these two traits had a similar relationship in both genetic groups, the gonadosomatic index was also very similar. This index represents the percentage ratio between the ovarian and the body weight and varied between 0.012 and 0.013% in improved and native guinea pigs, respectively. In other rodent species, the GSI was ~3.5 folds lower in rats (Ajala et al., 2013) and ~4 folds greater in mice (Wu et al., 2015) than in the guinea pigs in this study.
Overall, ovarian morphology, GSI, and the number of follicles on the ovarian surface did not vary between diestrus and proestrus in both genetic groups. However, the ovarian weight in improved guinea pigs was greater in diestrus than in proestrus. The weight and number of follicles in both ovaries did not change significantly between days 1, 6, 11, and 15 of the estrous cycle in nulliparous gui- nea pigs (Labhsetwar and Diamond, 1970). In that study, the weight of both ovaries varied between 90 and 120 mg, and the follicular count per animal between 70 and 90 follicles across the estrous cycle. In 2-4 month aged cyclic guinea pigs, the average length, width, and volume of ovaries was 4.9 ± 0.1 mm, 3.4 ± 0.1 mm, and 30.5 ± 1.7 mm3, respectively (Wang et al., 2019).
Histological examinations showed no difference in the number of each category of follicles between groups of guinea pigs, except for antral follicles whose number was significantly greater in proestrus of the improved guinea pigs. Even though the follicular count in all categories was similar between diestrus and proestrus in improved guinea pigs, the count of primordial and primary follicles and the total number of follicles in native guinea pigs was greater in diestrus than in proestrus. As follicular growth in guinea pigs occurs in two waves (Bland, 1980; Hamilton and Tam, 1990), a large variation in the number of follicles can be observed throughout the estrous cycle (Logothetopoulos et al., 1995). For instance, the number of nonantral follicles (two-layer of granulosa cell) on days 1, 3, 6, 9, 13, and 16 of the estrous cycle was 125.4, 97.3, 110, 67, 112.8 follicles respectively (Logothe- topoulos et al., 1995). Also, Hamilton and Tam (1990) observed the greatest number of growing follicles on days 0 and 9, and the lowest number on days 6 and 14 of the estrous cycle. Interestingly, the number of follicles around the time of proestrus in the Logothetopoulos et al. (1995) report was considerably lower than the equivalent period to diestrus, in accordance with the present study.
The number of follicles (total and per category) in this study is comparable with that reported by Labhsetwar and Diamond (1970). However, Peddie (1980), observed that the total number of antral follicles did not change significantly during the cycle and averaged 271.9 ± 37.8 on day 5, 232.2 ±29.9 on day 10 and 221.1 ± 24.9 on day 15; however, the maximum size of the follicles varied substantially. Although not specifically stated by Hamilton and Tam (1990), the total number of follicles ranged from 112 to 200 follicles during the estrous cycle of guinea pigs. These discrepancies in the total number of follicles in different studies are mainly due to methods of follicle counting and the number of slices analyzed. In the present study the objective was not to count as many follicles as possible per ovary but to count a sample that would allow the comparison of two genetic groups under the same criteria.
An interesting finding, not previously reported in guinea pigs was to observe the similarity of the zona pellucida thickness and the oocyte diameter between both groups of guinea pigs. It was also interesting to note that although the oocyte diameter did not vary between the oocyte categories in native and improved guinea pigs, category A oocytes (of greater quality) in both groups had a slightly thicker zona pellucida than the other oocyte categories. In cows, a lesser thickness of the zona pellucida was associated with a corpus luteum in the ovary from which the oocyte was collected, and with greater oocyte competence (Argudo et al., 2020). In guinea pigs, however, there is no evidence linking zona pellucida thickness with oocyte competence.
In the current study, the average diameter of oocytes collected mainly from antral follicles was 78.6 ± 0.31 µm. In a coincidence, a study in adult guinea pigs (Dunk in-Hartley strain with body weights between 562 and 750 g) determined an oocyte diameter of 78.9 ± 9.3 µm in antral follicles (Sadeu et al., 2007). A comparative study in several mammalian species reported oocyte diameters from antral follicles of 72.3, 79, 105.1, and 120 µm for mice, hamsters, pigs, and humans respectively (Griffin et al., 2006). In cows, the oocyte diameter in antral follicles was 124.8 µm (Argudo et al., 2020).
CONCLUSIONS
Ovaries from improved guinea pigs were heavier and longer than from natives. The gonadosomatic index and the number of follicles visible on the ovarian surface were similar between groups. With few exceptions, the number of follicles of different categories and the total follicles did not vary between genetic groups. There was a great similarity in zona pellucida thickness and oocyte diameter in native and improved guinea pigs. The proportion of class A, B, and C oocytes was similar between genetic groups. The likeness in the ovarian morphological characteristics of these two genetic groups suggests the use of similar reproductive management, with the further purpose of applying assisted reproduction techniques for improving productive and reproductive per- formance.












 uBio
uBio